Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels
- PMID: 19337257
- PMCID: PMC2670000
- DOI: 10.1038/sj.bjc.6604961
Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels
Abstract
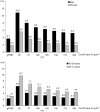
Immunochemical faecal occult blood testing (FIT) provides quantitative test results, which allows optimisation of the cut-off value for follow-up colonoscopy. We conducted a randomised population-based trial to determine test characteristics of FIT (OC-Sensor micro, Eiken, Japan) screening at different cut-off levels and compare these with guaiac-based faecal occult blood test (gFOBT) screening in an average risk population. A representative sample of the Dutch population (n=10 011), aged 50-74 years, was 1 : 1 randomised before invitation to gFOBT and FIT screening. Colonoscopy was offered to screenees with a positive gFOBT or FIT (cut-off 50 ng haemoglobin/ml). When varying the cut-off level between 50 and 200 ng ml(-1), the positivity rate of FIT ranged between 8.1% (95% CI: 7.2-9.1%) and 3.5% (95% CI: 2.9-4.2%), the detection rate of advanced neoplasia ranged between 3.2% (95% CI: 2.6-3.9%) and 2.1% (95% CI: 1.6-2.6%), and the specificity ranged between 95.5% (95% CI: 94.5-96.3%) and 98.8% (95% CI: 98.4-99.0%). At a cut-off value of 75 ng ml(-1), the detection rate was two times higher than with gFOBT screening (gFOBT: 1.2%; FIT: 2.5%; P<0.001), whereas the number needed to scope (NNscope) to find one screenee with advanced neoplasia was similar (2.2 vs 1.9; P=0.69). Immunochemical faecal occult blood testing is considerably more effective than gFOBT screening within the range of tested cut-off values. From our experience, a cut-off value of 75 ng ml(-1) provided an adequate positivity rate and an acceptable trade-off between detection rate and NNscope.
Figures
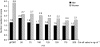
References
-
- Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, Pauly MP, Shlager L, Palitz AM, Zhao WK, Schwartz JS, Ransohoff DF, Selby JV (2007) Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst 99: 1462–1470 - PubMed
-
- Allison JE, Tekawa IS, Ransom LJ, Adrain AL (1996) A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 334: 155–159 - PubMed
-
- Brecht JG, Robra BP (1987) A graphic method of estimating the specificity of screening programmes from incomplete follow-up data. Methods Inf Med 26: 53–58 - PubMed
-
- Castiglione G, Grazzini G, Miccinesi G, Rubeca T, Sani C, Turco P, Zappa M (2002) Basic variables at different positivity thresholds of a quantitative immunochemical test for faecal occult blood. J Med Screen 9: 99–103 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

