Transforming growth factor beta as a therapeutic target in systemic sclerosis
- PMID: 19337284
- PMCID: PMC3959159
- DOI: 10.1038/nrrheum.2009.26
Transforming growth factor beta as a therapeutic target in systemic sclerosis
Abstract
Transforming growth factor beta (TGF-beta) is a pleiotropic cytokine with vital homeostatic functions. Aberrant TGF-beta expression is implicated in the pathogenesis of fibrosis in systemic sclerosis (SSc); thus, TGF-beta represents a molecular therapeutic target in this disease. Anti-TGF-beta monoclonal antibody has been evaluated in a small trial of early SSc, with disappointing results. Antibodies against the alphavbeta6 integrin that prevent latent TGF-beta activation, however, have shown promise in preclinical studies. Small-molecule inhibitors of TGF-beta-receptor activity are effective in animal models of fibrosis. Imatinib mesylate and related tyrosine kinase inhibitors also block TGF-beta pathways and abrogate fibrotic responses. The blocking of TGF-beta activity might lead to spontaneous immune activation, epithelial hyperplasia and impaired wound healing. Loss of immune tolerance is a potential concern in an autoimmune disease such as SSc. Novel insights from microarray-based gene expression analyses and studies of genetic polymorphisms in TGF-beta signaling could aid in identifying patients who are most likely to respond to anti-TGF-beta treatment. This intervention promises to have a major impact on the treatment of SSc. Concerns regarding efficacy and safety and whether biomarkers can indicate these features, questions regarding appropriate dosing and timing of therapy, and identification of potential responders are critical challenges ahead.
Figures
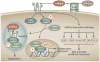

References
-
- Blobe GC, et al. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. - PubMed
-
- Prud’homme GJ. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest. 2007;87:1077–1091. - PubMed
-
- Feng XH, Derynck R. Specificity and versatility in TGF-beta signaling through SMADs. Annu Rev Cell Dev Biol. 2005;21:659–693. - PubMed
-
- Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782:197–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

