Characterization of voltage-gated Ca(2+) conductances in layer 5 neocortical pyramidal neurons from rats
- PMID: 19337371
- PMCID: PMC2659773
- DOI: 10.1371/journal.pone.0004841
Characterization of voltage-gated Ca(2+) conductances in layer 5 neocortical pyramidal neurons from rats
Abstract
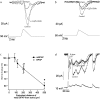
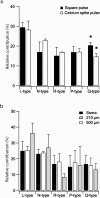
Neuronal voltage-gated Ca(2+) channels are involved in electrical signalling and in converting these signals into cytoplasmic calcium changes. One important function of voltage-gated Ca(2+) channels is generating regenerative dendritic Ca(2+) spikes. However, the Ca(2+) dependent mechanisms used to create these spikes are only partially understood. To start investigating this mechanism, we set out to kinetically and pharmacologically identify the sub-types of somatic voltage-gated Ca(2+) channels in pyramidal neurons from layer 5 of rat somatosensory cortex, using the nucleated configuration of the patch-clamp technique. The activation kinetics of the total Ba(2+) current revealed conductance activation only at medium and high voltages suggesting that T-type calcium channels were not present in the patches. Steady-state inactivation protocols in combination with pharmacology revealed the expression of R-type channels. Furthermore, pharmacological experiments identified 5 voltage-gated Ca(2+) channel sub-types - L-, N-, R- and P/Q-type. Finally, the activation of the Ca(2+) conductances was examined using physiologically derived voltage-clamp protocols including a calcium spike protocol and a mock back-propagating action potential (mBPAP) protocol. These experiments enable us to suggest the possible contribution of the five Ca(2+) channel sub-types to Ca(2+) current flow during activation under physiological conditions.
Conflict of interest statement
Figures





References
-
- Creutzfeldt OD. Generality of the functional structure of the neocortex. Naturwissenschaften. 1977;64:507–517. - PubMed
-
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. - PubMed
-
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. - PubMed
-
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–574. - PubMed

