The synovial sarcoma-associated SYT-SSX2 oncogene antagonizes the polycomb complex protein Bmi1
- PMID: 19337376
- PMCID: PMC2659801
- DOI: 10.1371/journal.pone.0005060
The synovial sarcoma-associated SYT-SSX2 oncogene antagonizes the polycomb complex protein Bmi1
Abstract
This study demonstrates deregulation of polycomb activity by the synovial sarcoma-associated SYT-SSX2 oncogene, also known as SS18-SSX2. Synovial sarcoma is a soft tissue cancer associated with a recurrent t(X:18) translocation event that generates one of two fusion proteins, SYT-SSX1 or SYT-SSX2. The role of the translocation products in this disease is poorly understood. We present evidence that the SYT-SSX2 fusion protein interacts with the polycomb repressive complex and modulates its gene silencing activity. SYT-SSX2 causes destabilization of the polycomb subunit Bmi1, resulting in impairment of polycomb-associated histone H2A ubiquitination and reactivation of polycomb target genes. Silencing by polycomb complexes plays a vital role in numerous physiological processes. In recent years, numerous reports have implicated gain of polycomb silencing function in several cancers. This study provides evidence that, in the appropriate context, expression of the SYT-SSX2 oncogene leads to loss of polycomb function. It challenges the notion that cancer is solely associated with an increase in polycomb function and suggests that any imbalance in polycomb activity could drive the cell toward oncogenesis. These findings provide a mechanism by which the SYT-SSX2 chimera may contribute to synovial sarcoma pathogenesis.
Conflict of interest statement
Figures
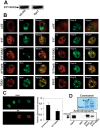

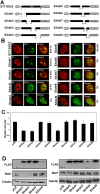
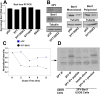

References
-
- Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. - PubMed
-
- Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. - PubMed
-
- van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet. 1999;23:474–478. - PubMed
-
- Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. - PubMed
-
- Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

