Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes
- PMID: 19337387
- PMCID: PMC2662587
- DOI: 10.1111/j.1752-8062.2008.00026.x
Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes
Abstract
Objectives: Chronic subacute inflammation is implicated in the pathogenesis of insulin resistance and type 2 diabetes. Salicylates were shown years ago to lower glucose and more recently to inhibit NF-kappaB activity. Salsalate, a prodrug form of salicylate, has seen extensive clinical use and has a favorable safety profile. We studied the efficacy of salsalate in reducing glycemia and insulin resistance and potential mechanisms of action to validate NF-kappaB as a potential pharmacologic target in diabetes.
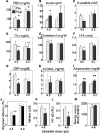
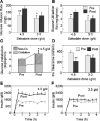
Methods and results: In open label studies, both high (4.5 g/d) and standard (3.0 g/d) doses of salsalate reduced fasting and postchallenge glucose levels after 2 weeks of treatment. Salsalate increased glucose utilization during euglycemic hyperinsulinemic clamps, by approximately 50% and 15% at the high and standard doses, respectively, and insulin clearance was decreased. Dose-limiting tinnitus occurred only at the higher dose. In a third, double-masked, placebo-controlled trial, 1 month of salsalate at maximum tolerable dose (no tinnitus) improved fasting and postchallenge glucose levels. Circulating free fatty acids were reduced and adiponectin increased in all treated subjects.
Conclusions: These data demonstrate that salsalate improves in vivo glucose and lipid homeostasis, and support targeting of inflammation and NF-kappaB as a therapeutic approach in type 2 diabetes.
Keywords: adiponectin; glucose; inflammation; insulin resistance; salicylate; salsalate; type 2 diabetes.
Figures



References
-
- Tataranni PA, Ortega E. A burning question: does an adipokine‐induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005; 54(4): 917–927. - PubMed
-
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444(7121): 860–867. - PubMed
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352(16): 1685–1695. - PubMed
-
- Hansson GK, Libby P. The immune response in atherosclerosis: a double‐edged sword. Nat Rev Immunol. 2006; 6(7): 508–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

