Safety and efficacy of nateglinide/metformin combination therapy in the treatment of type 2 diabetes
- PMID: 19337530
- PMCID: PMC2663444
- DOI: 10.2147/vhrm.s2718
Safety and efficacy of nateglinide/metformin combination therapy in the treatment of type 2 diabetes
Abstract
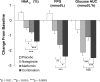
The increasing prevalence of type 2 diabetes provides impetus for both development of new drugs to improve glycemic control and for reconsideration of treatment strategies with existing agents. Combination therapy with complementary drug classes that act on different aspects of glycemic control has been a particularly effective strategy. This work reviews the published literature reporting efficacy and safety/tolerability of nateglinide, a rapid-onset insulinotropic agent with a predominant effect to reduce postprandial glucose, when combined with metformin, a first-line agent that suppresses hepatic glucose production and thereby reduces fasting plasma glucose. The nateglinide/metformin combination has consistently been found to be both efficacious and well tolerated, whether given as initial combination therapy in drug-naïve patients or when added to metformin monotherapy. Maximum efficacy (Delta glycosylated hemoglobin [HbA(1c)]= -1.4% to -1.9%, sustained for up to 2 years of treatment) was seen in studies of drug-naïve patients in whom pharmacotherapy was initiated with the combination of nateglinide and metformin, and modest reductions in HbA(1c) (Delta = -0.5% to -1.2%, sustained for up to 24 weeks) were found when nateglinide was added to ongoing metformin monotherapy.
Conclusion: the combination of nateglinide and metformin provides a sustained degree of glycemic control not achievable with either agent given as monotherapy.
Keywords: combination therapy; metformin; nateglinide; postprandial hyperglycemia; type 2 diabetes.
Figures

Similar articles
-
Efficacy and tolerability of initial combination therapy with nateglinide and metformin in treatment-naïve patients with type 2 diabetes.Curr Med Res Opin. 2004 Jun;20(6):883-9. doi: 10.1185/030079903125003881. Curr Med Res Opin. 2004. PMID: 15200747 Clinical Trial.
-
Efficacy and safety of combination therapy: repaglinide plus metformin versus nateglinide plus metformin.Diabetes Care. 2003 Jul;26(7):2063-8. doi: 10.2337/diacare.26.7.2063. Diabetes Care. 2003. PMID: 12832314 Clinical Trial.
-
Nateglinide, alone or in combination with metformin, is effective and well tolerated in treatment-naïve elderly patients with type 2 diabetes.Diabetes Obes Metab. 2008 Aug;10(8):652-60. doi: 10.1111/j.1463-1326.2007.00792.x. Epub 2007 Oct 15. Diabetes Obes Metab. 2008. PMID: 17941876 Clinical Trial.
-
[Nateglinide].Nihon Rinsho. 2002 Sep;60 Suppl 9:371-5. Nihon Rinsho. 2002. PMID: 12387020 Review. Japanese. No abstract available.
-
Nateglinide--current and future role in the treatment of patients with type 2 diabetes mellitus.Int J Clin Pract. 2005 Oct;59(10):1218-28. doi: 10.1111/j.1368-5031.2005.00669.x. Int J Clin Pract. 2005. PMID: 16178991 Review.
Cited by
-
Rule-based multi-scale simulation for drug effect pathway analysis.BMC Med Inform Decis Mak. 2013;13 Suppl 1(Suppl 1):S4. doi: 10.1186/1472-6947-13-S1-S4. Epub 2013 Apr 5. BMC Med Inform Decis Mak. 2013. PMID: 23566173 Free PMC article.
-
Nateglinide in combination with metformin in Chinese patients with type 2 diabetes mellitus: a post-marketing surveillance study.Clin Drug Investig. 2013 Mar;33(3):185-91. doi: 10.1007/s40261-013-0054-4. Clin Drug Investig. 2013. PMID: 23338975
-
Efficacy and safety of liraglutide versus sitagliptin both in combination with metformin in patients with type 2 diabetes: A systematic review and meta-analysis.Medicine (Baltimore). 2017 Sep;96(39):e8161. doi: 10.1097/MD.0000000000008161. Medicine (Baltimore). 2017. PMID: 28953663 Free PMC article.
References
-
- Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9. - PubMed
-
- Baron MA. Comparison of repaglinide and nateglinide in combination with metformin: response to Raskin et al. Diabetes Care. 2003;26:3361–2. - PubMed
-
- Centers for Disease Control and Prevention National diabetes fact sheet: United States 2005. [online]. Accessed September 11, 2007.URL: http://apps.nccd.cdc.gov/ddtstrs/template/ndfs_2005.pdf
-
- Ceriello A. The post-prandial state and cardiovascular disease: relevance to diabetes mellitus. Diabetes Metab Res Rev. 2000;16:125–32. - PubMed
-
- Cook MN, Girman CJ, Stein PP, et al. Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with type 2 diabetes in UK primary care. Diabet Med. 2007;24:350–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

