Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized (1)H MRS study
- PMID: 19338025
- PMCID: PMC2732191
- DOI: 10.1002/nbm.1385
Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized (1)H MRS study
Abstract
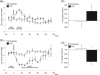
Acute phencyclidine (PCP) administration mimics some aspects of schizophrenia in rats, such as behavioral alterations, increased dopaminergic activity and prefrontal cortex dysfunction. In this study, we used single-voxel (1)H-MRS to investigate neurochemical changes in rat prefrontal cortex in vivo before and after an acute injection of PCP. A short-echo time sequence (STEAM) was used to acquire spectra in a 32-microL voxel positioned in the prefrontal cortex area of 12 rats anesthetized with isoflurane. Data were acquired for 30 min before and for 140 min after a bolus of PCP (10 mg/kg, n = 6) or saline (n = 6). Metabolites were quantified with the LCModel. Time courses for 14 metabolites were obtained with a temporal resolution of 10 min. The glutamine/glutamate ratio was significantly increased after PCP injection (p < 0.0001, pre- vs. post-injection), while the total concentration of these two metabolites remained constant. Glucose was transiently increased (+70%) while lactate decreased after the injection (both p < 0.0001). Lactate, but not glucose and glutamine, returned to baseline levels after 140 min. These results show that an acute injection of PCP leads to changes in glutamate and glutamine concentrations, similar to what has been observed in schizophrenic patients, and after ketamine administration in humans. MRS studies of this pharmacological rat model may be useful for assessing the effects of potential anti-psychotic drugs in vivo.
2009 John Wiley & Sons, Ltd.
Figures


References
-
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. - PubMed
-
- Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. - PubMed
-
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:1071–1079. - PubMed
-
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. - PubMed
-
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu. Rev. Pharmacol. Toxicol. 2001;41:237–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

