Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice
- PMID: 19338032
- PMCID: PMC3767389
- DOI: 10.1002/jor.20886
Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice
Abstract
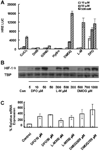
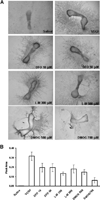
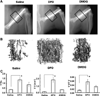
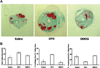
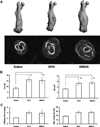
Skeletal trauma and impaired skeletal healing is commonly associated with diminished vascularity. Hypoxia inducible factor alpha (HIF-1) is a key transcription factor responsible for activating angiogenic factors during development and tissue repair. Small molecule inhibitors of the prolyl hydroxylase enzyme (PHD), the key enzyme responsible for degrading HIF-1, have been shown to activate HIF-1, and are effective in inducing angiogenesis. Here we examined the effects of several commercially available PHD inhibitors on bone marrow mesenchymal stromal cells (MSCs) in vitro and in a stabilized fracture model in vivo. Three PHD inhibitors [Desferrioxamine (DFO), L-mimosine (L-mim), and Dimethyloxalylglycine (DMOG)] effectively activated a HIF-1 target reporter, induced expression of vascular endothelial growth factor (VEGF) mRNA in vitro, and increased capillary sprouting in a functional angiogenesis assay. DFO and DMOG were applied by direct injection at the fracture site in a stabilized murine femur fracture model. PHD inhibition increased the vascularity at 14 days and increased callus size as assessed by microCT at 28 days. These results suggest that HIF activation is a viable approach to increase vascularity and bone formation following skeletal trauma.
(c) 2009 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures
References
-
- U.S. Bone and Joint Decade. The burden of musculoskeletal diseases in the United States. Rosemont, IL: AAOS; 2008. p. 268.
-
- Eckardt H, Ding M, Lind M, et al. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J Bone Joint Surg Br. 2005;87:1434–1438. - PubMed
-
- Murnaghan M, Li G, Marsh DR. Nonsteroidal antiinflammatory drug-induced fracture nonunion: an inhibition of angiogenesis? J Bone Joint Surg Am. 2006;88(Suppl 3):140–147. - PubMed
-
- Li R, Stewart DJ, von Schroeder HP, et al. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J Orthop Res. 2008;27:8–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

