AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location
- PMID: 19338055
- PMCID: PMC2677127
- DOI: 10.1002/ana.21589
AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location
Abstract
Objective: To report the clinical and immunological features of a novel autoantigen related to limbic encephalitis (LE) and the effect of patients' antibodies on neuronal cultures.
Methods: We conducted clinical analyses of 10 patients with LE. Immunoprecipitation and mass spectrometry were used to identify the antigens. Human embryonic kidney 293 cells expressing the antigens were used in immunocytochemistry and enzyme-linked immunoabsorption assay. The effect of patients' antibodies on cultures of live rat hippocampal neurons was determined with confocal microscopy.
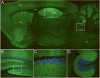
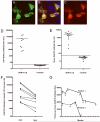
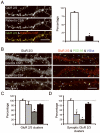
Results: Median age was 60 (38-87) years; 9 were women. Seven had tumors of the lung, breast, or thymus. Nine patients responded to immunotherapy or oncological therapy, but neurological relapses, without tumor recurrence, were frequent and influenced the long-term outcome. One untreated patient died of LE. All patients had antibodies against neuronal cell surface antigens that by immunoprecipitation were found to be the glutamate receptor 1 (GluR1) and GluR2 subunits of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR). Human embryonic kidney 293 cells expressing GluR1/2 reacted with all patients' sera or cerebrospinal fluid, providing a diagnostic test for the disorder. Application of antibodies to cultures of neurons significantly decreased the number of GluR2-containing AMPAR clusters at synapses with a smaller decrease in overall AMPAR cluster density; these effects were reversed after antibody removal.
Interpretation: Antibodies to GluR1/2 associate with LE that is often paraneoplastic, treatment responsive, and has a tendency to relapse. Our findings support an antibody-mediated pathogenesis in which patients' antibodies alter the synaptic localization and number of AMPARs.
Figures


References
-
- Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain. 1968;91:481–496. - PubMed
-
- Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123(Pt 7):1481–1494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 CA089054/CA/NCI NIH HHS/United States
- T32 AG023481/AG/NIA NIH HHS/United States
- R56 MH057683/MH/NIMH NIH HHS/United States
- 2R56CA089054/CA/NCI NIH HHS/United States
- R01 MH057683/MH/NIMH NIH HHS/United States
- R01 CA107192/CA/NCI NIH HHS/United States
- R01CA107192/CA/NCI NIH HHS/United States
- NSR56-45986/PHS HHS/United States
- AG023481/AG/NIA NIH HHS/United States
- NSR01-45986/PHS HHS/United States
- F31 MH083395/MH/NIMH NIH HHS/United States
- R21 MH057683/MH/NIMH NIH HHS/United States
- F31MH083395/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

