Electronic control of DNA polymerase binding and unbinding to single DNA molecules
- PMID: 19338283
- PMCID: PMC2708927
- DOI: 10.1021/nn9000897
Electronic control of DNA polymerase binding and unbinding to single DNA molecules
Abstract
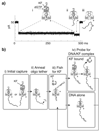
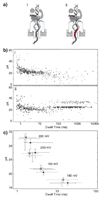
DNA polymerases catalyze template-dependent genome replication. The assembly of a high affinity ternary complex between these enzymes, the double strand-single strand junction of their DNA substrate, and the deoxynucleoside triphosphate (dNTP) complementary to the first template base in the polymerase active site is essential to this process. We present a single molecule method for iterative measurements of DNA-polymerase complex assembly with high temporal resolution, using active voltage control of individual DNA substrate molecules tethered noncovalently in an alpha-hemolysin nanopore. DNA binding states of the Klenow fragment of Escherichia coli DNA polymerase I (KF) were diagnosed based upon their ionic current signature, and reacted to with submillisecond precision to execute voltage changes that controlled exposure of the DNA substrate to KF and dNTP. Precise control of exposure times allowed measurements of DNA-KF complex assembly on a time scale that superimposed with the rate of KF binding. Hundreds of measurements were made with a single tethered DNA molecule within seconds, and dozens of molecules can be tethered within a single experiment. This approach allows statistically robust analysis of the assembly of complexes between DNA and RNA processing enzymes and their substrates at the single molecule level.
Figures




Comment in
-
Nanopore-based biosensors: the interface between ionics and electronics.ACS Nano. 2009 Apr 28;3(4):775-9. doi: 10.1021/nn900336j. ACS Nano. 2009. PMID: 19397344
References
-
- Joyce CM, Steitz TA. Function and Structure Relationships in DNA Polymerases. Anal. Rev. Biochem. 1994;63:777–822. - PubMed
-
- Joyce CM, Benkovic SJ. DNA Polymerase Fidelity: Kinetics, Structure, and Checkpoints. Biochemistry. 2004;43:14317–14324. - PubMed
-
- Brautigam CA, Steitz TA. Structural and Functional Insights Provided by Crystal Structures of DNA Polymerases and their Substrate Complexes. Current Opinion in Structural Biology. 1998;8:54–63. - PubMed
-
- Joyce CM, Potapova O, Delucia A, Huang X, Basu V, Grindley NDF. Fingers-Closing and Other Rapid Conformational Changes in DNA Polymerase I (Klenow Fragment) and Their Role in Nucleotide Selectivity. Biochemistry. 2008;47:6103–6116. - PubMed
-
- Doublié S, Tabor S, Long AM, Richardson CC, Ellenberger T. Crystal Structure of a Bacteriophage T7 DNA Replication Complex at 2.2 Å Resolution. Nature. 1998;391:251–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

