Transient overexpression of sonic hedgehog alters the architecture and mechanical properties of trabecular bone
- PMID: 19338448
- PMCID: PMC3276343
- DOI: 10.1359/jbmr.090313
Transient overexpression of sonic hedgehog alters the architecture and mechanical properties of trabecular bone
Abstract
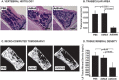
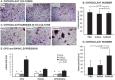
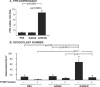
Bone formation and remodeling involve coordinated interactions between osteoblasts and osteoclasts through signaling networks involving a variety of molecular pathways. We hypothesized that overexpression of Sonic hedgehog (Shh), a morphogen with a crucial role in skeletal development, would stimulate osteoblastogenesis and bone formation in adult animals in vivo. Systemic administration of adenovirus expressing the N-terminal form of Shh into adult mice resulted in a primary increase in osteoblasts and their precursors. Surprisingly, however, this was associated with altered trabecular morphology, decreased bone volume, and decreased compressive strength in the vertebrae. Whereas no change was detected in the number of osteoclast precursors, bone marrow stromal cells from Shh-treated mice showed enhanced osteoclastogenic potential in vitro. These effects were mediated by the PTH/PTH-related protein (PTHrP) pathway as evidenced by increased sensitivity to PTH stimulation and upregulation of the PTH/PTHrP receptor (PPR). Together, these data show that Shh has stimulatory effects on osteoprogenitors and osteoblasts in adult animals in vivo, which results in bone remodeling and reduced bone strength because of a secondary increase in osteoclastogenesis.
Figures



Similar articles
-
Endogenous parathyroid hormone-related protein compensates for the absence of parathyroid hormone in promoting bone accrual in vivo in a model of bone marrow ablation.J Bone Miner Res. 2013 Sep;28(9):1898-911. doi: 10.1002/jbmr.2000. J Bone Miner Res. 2013. PMID: 23716486
-
Parathyroid hormone-related peptide is required for increased trabecular bone volume in parathyroid hormone-null mice.Endocrinology. 2004 Aug;145(8):3554-62. doi: 10.1210/en.2003-1695. Epub 2004 Apr 16. Endocrinology. 2004. PMID: 15090463
-
Sonic hedgehog expands diaphyseal trabecular bone altering bone marrow niche and lymphocyte compartment.Mol Ther. 2009 Aug;17(8):1442-52. doi: 10.1038/mt.2009.102. Epub 2009 May 12. Mol Ther. 2009. PMID: 19436267 Free PMC article.
-
[Hormones and osteoporosis update. Histological aspects on the action of parathyroid hormone (PTH) and PTH-related peptide (PTHrP) on bone and cartilage].Clin Calcium. 2009 Jul;19(7):935-43. Clin Calcium. 2009. PMID: 19567988 Review. Japanese.
-
Morphology and biochemistry of bone remodeling: possible control by vitamin D, parathyroid hormone, and other substances.Lab Invest. 1988 Oct;59(4):418-42. Lab Invest. 1988. PMID: 3050272 Review.
Cited by
-
The Hedgehog signalling pathway in bone formation.Int J Oral Sci. 2015 Jun 26;7(2):73-9. doi: 10.1038/ijos.2015.14. Int J Oral Sci. 2015. PMID: 26023726 Free PMC article. Review.
-
Morphoproteomics provides support for TGF-β pathway signaling in the osteoclastogenesis and immune dysregulation of osteolytic Langerhans cell histiocytosis.Int J Clin Exp Pathol. 2012;5(6):503-11. Epub 2012 Jul 29. Int J Clin Exp Pathol. 2012. PMID: 22949932 Free PMC article.
-
Pituitary adenylate cyclase-activating polypeptide (PACAP) signalling enhances osteogenesis in UMR-106 cell line.J Mol Neurosci. 2014 Nov;54(3):555-73. doi: 10.1007/s12031-014-0389-1. Epub 2014 Aug 12. J Mol Neurosci. 2014. PMID: 25112418
-
Could use of Selective Serotonin Reuptake Inhibitors During Lactation Cause Persistent Effects on Maternal Bone?J Mammary Gland Biol Neoplasia. 2018 Jun;23(1-2):5-25. doi: 10.1007/s10911-018-9390-6. Epub 2018 Mar 30. J Mammary Gland Biol Neoplasia. 2018. PMID: 29603039 Review.
-
Roles for Hedgehog signaling in adult organ homeostasis and repair.Development. 2014 Sep;141(18):3445-57. doi: 10.1242/dev.083691. Development. 2014. PMID: 25183867 Free PMC article. Review.
References
-
- Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D, Xue A, Montag AG, Luu HH, Haydon RC, He TC. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. - PubMed
-
- Hadjidakis DJ. Androulakis II 2006 Bone remodeling. Ann NY Acad Sci. 1092:385–396. - PubMed
-
- Favus M, editor. ASBMR Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6th ed. Chicago, IL, USA: University of Chicago Medical Center; 2006.
-
- Ehlen HW, Buelens LA, Vortkamp A. Hedgehog signaling in skeletal development. Birth Defects Res C Embryo Today. 2006;78:267–279. - PubMed
-
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

