FRS2 via fibroblast growth factor receptor 1 is required for platelet-derived growth factor receptor beta-mediated regulation of vascular smooth muscle marker gene expression
- PMID: 19339244
- PMCID: PMC2708892
- DOI: 10.1074/jbc.M809399200
FRS2 via fibroblast growth factor receptor 1 is required for platelet-derived growth factor receptor beta-mediated regulation of vascular smooth muscle marker gene expression
Erratum in
- J Biol Chem. 2012 Jan 6;287(2):1609
Abstract
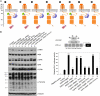
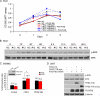
Vascular smooth muscle cells (VSMC) exhibit phenotypic plasticity and change from a quiescent contractile phenotype to a proliferative synthetic phenotype during physiological arteriogenesis and pathological conditions such as atherosclerosis and restenosis. Platelet-derived growth factor (PDGF)-BB is a potent inducer of the VSMC synthetic phenotype; however, much less is known about the role of fibroblast growth factor-2 (FGF2) in this process. Here, we show using signal transduction mutants of FGF receptor 1 (FGFR1) expressed in rat VSMC that the adaptor protein FRS2 is essential for FGFR1-mediated phenotypic modulation and down-regulation of VSMC smooth muscle alpha-actin (SMA) gene expression. In addition, we show that PDGF-BB and FGF2 act synergistically to induce cell proliferation and down-regulate SMA and SM22alpha in VSMC. Furthermore, we show that PDGF-BB induces tyrosine phosphorylation of FGFR1 and that this phosphorylation is mediated by PDGF receptor-beta (PDGFRbeta), but not c-Src. We demonstrate that FRS2 co-immunoprecipitates with PDGFRbeta in a complex that requires FGFR1 and that both the extracellular and the intracellular domains of FGFR1 are required for association with PDGFRbeta, whereas the cytoplasmic domain of FGFR1 is required for FRS2 association with the FGFR1-PDGFRbeta complex. Knockdown of FRS2 in VSMC by RNA interference inhibited PDGF-BB-mediated down-regulation of SMA and SM22alpha without affecting PDGF-BB mediated cell proliferation or ERK activation. Together, these data support the notion that PDGFRbeta down-regulates SMA and SM22alpha through formation of a complex that requires FGFR1 and FRS2 and prove novel insight into VSMC phenotypic plasticity.
Figures







References
-
- Owens G. K. ( 2007) Novartis Found. Symp. 283, 174– 193 - PubMed
-
- Rzucidlo E. M., Martin K. A., Powell R. J. ( 2007) J. Vasc. Surg. 45, ( Suppl. A) 25– 32 - PubMed
-
- Millette E., Rauch B. H., Kenagy R. D., Daum G., Clowes A. W. ( 2006) Trends Cardiovasc. Med. 16, 25– 28 - PubMed
-
- Lindner V. ( 1995) Z. Kardiol. 84, ( Suppl. 4) 137– 144 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

