The relation between inflammation and neurodegeneration in multiple sclerosis brains
- PMID: 19339255
- PMCID: PMC2677799
- DOI: 10.1093/brain/awp070
The relation between inflammation and neurodegeneration in multiple sclerosis brains
Abstract
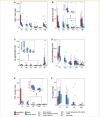
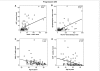
Some recent studies suggest that in progressive multiple sclerosis, neurodegeneration may occur independently from inflammation. The aim of our study was to analyse the interdependence of inflammation, neurodegeneration and disease progression in various multiple sclerosis stages in relation to lesional activity and clinical course, with a particular focus on progressive multiple sclerosis. The study is based on detailed quantification of different inflammatory cells in relation to axonal injury in 67 multiple sclerosis autopsies from different disease stages and 28 controls without neurological disease or brain lesions. We found that pronounced inflammation in the brain is not only present in acute and relapsing multiple sclerosis but also in the secondary and primary progressive disease. T- and B-cell infiltrates correlated with the activity of demyelinating lesions, while plasma cell infiltrates were most pronounced in patients with secondary progressive multiple sclerosis (SPMS) and primary progressive multiple sclerosis (PPMS) and even persisted, when T- and B-cell infiltrates declined to levels seen in age matched controls. A highly significant association between inflammation and axonal injury was seen in the global multiple sclerosis population as well as in progressive multiple sclerosis alone. In older patients (median 76 years) with long-disease duration (median 372 months), inflammatory infiltrates declined to levels similar to those found in age-matched controls and the extent of axonal injury, too, was comparable with that in age-matched controls. Ongoing neurodegeneration in these patients, which exceeded the extent found in normal controls, could be attributed to confounding pathologies such as Alzheimer's or vascular disease. Our study suggests a close association between inflammation and neurodegeneration in all lesions and disease stages of multiple sclerosis. It further indicates that the disease processes of multiple sclerosis may die out in aged patients with long-standing disease.
Figures



References
-
- Anderson VM, Fox NC, Miller DH. Magnetic resonance imaging measures of brain atrophy in multiple sclerosis. J Magn Reson Imaging. 2006;23:605–18. - PubMed
-
- Bauer J, Elger CE, Hans VH, Schramm J, Urbach H, Lassmann H, et al. Astrocytes are a specific immunological target in Rasmussen's encephalitis. Ann Neurol. 2007;62:67–80. - PubMed
-
- Bergers E, Bot JC, De Groot CJ, Polman CH, Nijeholt GJ, Castelijns JA, et al. Axonal damage in the spinal cord of MS patients occurs largely independent of T2 MRI lesions. Neurology. 2002;59:1766–71. - PubMed
-
- Bielekova B, Kadom N, Fisher E, Jeffries N, Ohayon J, Richert N, et al. MRI as a marker for disease heterogeneity in multiple sclerosis. Neurology. 2005;65:1071–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

