Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism
- PMID: 19339281
- PMCID: PMC2688549
- DOI: 10.1091/mbc.e08-10-1079
Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism
Abstract
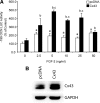
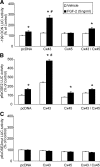
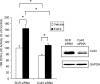
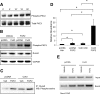
In this study, we examine the role of the gap junction protein, connexin43 (Cx43), in the transcriptional response of osteocalcin to fibroblast growth factor 2 (FGF2) in MC3T3 osteoblasts. By luciferase reporter assays, we identify that the osteocalcin transcriptional response to FGF2 is markedly increased by overexpression of Cx43, an effect that is mediated by Runx2 via its OSE2 cognate element, but not by a previously identified connexin-responsive Sp1/Sp3-binding element. Furthermore, disruption of Cx43 function with Cx43 siRNAs or overexpression of connexin45 markedly attenuates the response to FGF2. Inhibition of protein kinase C delta (PKCdelta) with rottlerin or siRNA-mediated knockdown abrogates the osteocalcin response to FGF2. Additionally, we show that upon treatment with FGF2, PKCdelta translocates to the nucleus, PKCdelta and Runx2 are phosphorylated and these events are enhanced by Cx43 overexpression, suggesting that the degree of activation is enhanced by increased Cx43 levels. Indeed, chromatin immunoprecipitations of the osteocalcin proximal promoter with antibodies against Runx2 demonstrate that the recruitment of Runx2 to the osteocalcin promoter in response to FGF2 treatment is dramatically enhanced by Cx43 overexpression. Thus, Cx43 plays a critical role in regulating the ability of osteoblasts to respond to FGF2 by impacting PKCdelta and Runx2 function.
Figures



Similar articles
-
ERK acts in parallel to PKCδ to mediate the connexin43-dependent potentiation of Runx2 activity by FGF2 in MC3T3 osteoblasts.Am J Physiol Cell Physiol. 2012 Apr 1;302(7):C1035-44. doi: 10.1152/ajpcell.00262.2011. Epub 2012 Jan 25. Am J Physiol Cell Physiol. 2012. PMID: 22277757 Free PMC article.
-
The regulation of runt-related transcription factor 2 by fibroblast growth factor-2 and connexin43 requires the inositol polyphosphate/protein kinase Cδ cascade.J Bone Miner Res. 2013 Jun;28(6):1468-77. doi: 10.1002/jbmr.1867. J Bone Miner Res. 2013. PMID: 23322705 Free PMC article.
-
An intact connexin43 is required to enhance signaling and gene expression in osteoblast-like cells.J Cell Biochem. 2013 Nov;114(11):2542-50. doi: 10.1002/jcb.24603. J Cell Biochem. 2013. PMID: 23744706 Free PMC article.
-
Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2.J Biol Chem. 2002 Sep 27;277(39):36181-7. doi: 10.1074/jbc.M206057200. Epub 2002 Aug 28. J Biol Chem. 2002. PMID: 12110689
-
FGF2 stimulation of the pyrophosphate-generating enzyme, PC-1, in pre-osteoblast cells is mediated by RUNX2.J Bone Miner Res. 2009 Apr;24(4):652-62. doi: 10.1359/jbmr.081213. J Bone Miner Res. 2009. PMID: 19049325 Free PMC article.
Cited by
-
Connexin43 in Musculoskeletal System: New Targets for Development and Disease Progression.Aging Dis. 2022 Dec 1;13(6):1715-1732. doi: 10.14336/AD.2022.0421. eCollection 2022 Dec 1. Aging Dis. 2022. PMID: 36465186 Free PMC article.
-
Connexin43 regulates osteoprotegerin expression via ERK1/2 -dependent recruitment of Sp1.Biochem Biophys Res Commun. 2019 Feb 12;509(3):728-733. doi: 10.1016/j.bbrc.2018.12.173. Epub 2019 Jan 7. Biochem Biophys Res Commun. 2019. PMID: 30626485 Free PMC article.
-
Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures.Proc Natl Acad Sci U S A. 2011 Sep 27;108(39):16277-82. doi: 10.1073/pnas.1110286108. Epub 2011 Sep 12. Proc Natl Acad Sci U S A. 2011. PMID: 21911383 Free PMC article.
-
Gap junctional regulation of signal transduction in bone cells.FEBS Lett. 2014 Apr 17;588(8):1315-21. doi: 10.1016/j.febslet.2014.01.025. Epub 2014 Jan 28. FEBS Lett. 2014. PMID: 24486014 Free PMC article. Review.
-
Gap junctions and hemichannels in signal transmission, function and development of bone.Biochim Biophys Acta. 2012 Aug;1818(8):1909-18. doi: 10.1016/j.bbamem.2011.09.018. Epub 2011 Sep 22. Biochim Biophys Acta. 2012. PMID: 21963408 Free PMC article. Review.
References
-
- Ahmed A., Plevin R., Shoaibi M. A., Fountain S. A., Ferriani R. A., Smith S. K. Basic FGF activates phospholipase D in endothelial cells in the absence of inositol-lipid hydrolysis. Am. J. Physiol. 1994;266:C206–C212. - PubMed
-
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of the gap junction proteins. J. Membr. Biol. 1990;116:187–194. - PubMed
-
- Boudreaux J. M., Towler D. A. Synergistic induction of osteocalcin gene expression: identification of a bipartite element conferring fibroblast growth factor 2 and cyclic AMP responsiveness in the rat osteocalcin promoter. J. Biol. Chem. 1996;271:7508–7515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

