Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity
- PMID: 19339487
- PMCID: PMC2691038
- DOI: 10.1128/CVI.00007-09
Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity
Abstract
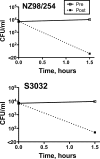
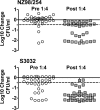
The binding of complement factor H (fH) to meningococci was recently found to be specific for human fH. Therefore, passive protective antibody activity measured in animal models of meningococcal bacteremia may overestimate protection in humans, since in the absence of bound fH, complement activation is not downregulated. We developed an ex vivo model of meningococcal bacteremia using nonimmune human blood to measure the passive protective activity of stored sera from 36 adults who had been immunized with an investigational meningococcal multicomponent recombinant protein vaccine. Before immunization, human complement-mediated serum bactericidal activity (SBA) titers of > or = 1:4 against group B strains H44/76, NZ98/254, and S3032 were present in 19, 11, and 8% of subjects, respectively; these proportions increased to 97, 22, and 36%, respectively, 1 month after dose 3 (P < 0.01 for H44/76 and S3032). Against the two SBA-resistant strains, NZ98/254 and S3032, passive protective titers of > or = 1:4 were present in 11 and 42% of sera before immunization, respectively, and these proportions increased to 61 and 94% after immunization (P < 0.001 for each strain). Most of the sera with SBA titers of <1:4 and passive protective activity showed a level of killing in the whole-blood assay (>1 to 2 log(10) decreases in CFU/ml during a 90-min incubation) similar to that of sera with SBA titers of > or = 1:4. In conclusion, passive protective activity was 2.6- to 2.8-fold more frequent than SBA after immunization. The ability of SBA-negative sera to kill Neisseria meningitidis in human blood where fH is bound to the bacteria provides further evidence that SBA titers of > or = 1:4 measured with human complement may underestimate meningococcal immunity.
Figures



Similar articles
-
A glycoconjugate vaccine for Neisseria meningitidis induces antibodies in human infants that afford protection against meningococcal bacteremia in a neonate rat challenge model.Infect Immun. 2002 Dec;70(12):6576-82. doi: 10.1128/IAI.70.12.6576-6582.2002. Infect Immun. 2002. PMID: 12438327 Free PMC article. Clinical Trial.
-
Age-related disparity in functional activities of human group C serum anticapsular antibodies elicited by meningococcal polysaccharide vaccine.Infect Immun. 2003 Jan;71(1):275-86. doi: 10.1128/IAI.71.1.275-286.2003. Infect Immun. 2003. PMID: 12496177 Free PMC article.
-
Naturally acquired passive protective activity against Neisseria meningitidis Group C in the absence of serum bactericidal activity.Infect Immun. 2004 Oct;72(10):5903-9. doi: 10.1128/IAI.72.10.5903-5909.2004. Infect Immun. 2004. PMID: 15385492 Free PMC article.
-
Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease.Vaccine. 2009 Jun 24;27 Suppl 2:B112-6. doi: 10.1016/j.vaccine.2009.04.065. Epub 2009 May 21. Vaccine. 2009. PMID: 19464093 Review.
-
Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease.Vaccine. 2009 Jun 24;27 Suppl 2(Suppl 2):B117-25. doi: 10.1016/j.vaccine.2009.04.066. Epub 2009 May 23. Vaccine. 2009. PMID: 19477054 Free PMC article. Review.
Cited by
-
Vaccines, reverse vaccinology, and bacterial pathogenesis.Cold Spring Harb Perspect Med. 2013 May 1;3(5):a012476. doi: 10.1101/cshperspect.a012476. Cold Spring Harb Perspect Med. 2013. PMID: 23637311 Free PMC article.
-
Effect of factor H-binding protein sequence variation on factor H binding and survival of Neisseria meningitidis in human blood.Infect Immun. 2011 Jan;79(1):353-9. doi: 10.1128/IAI.00849-10. Epub 2010 Nov 1. Infect Immun. 2011. PMID: 21041484 Free PMC article.
-
Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates.Vaccine. 2010 Feb 25;28(9):2122-9. doi: 10.1016/j.vaccine.2009.12.027. Epub 2009 Dec 29. Vaccine. 2010. PMID: 20044056 Free PMC article.
-
Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants.Hum Vaccin Immunother. 2016 May 3;12(5):1300-10. doi: 10.1080/21645515.2015.1136040. Epub 2016 Feb 1. Hum Vaccin Immunother. 2016. PMID: 26829877 Free PMC article. Review.
-
Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults.Blood. 2017 Aug 17;130(7):891-899. doi: 10.1182/blood-2017-05-781450. Epub 2017 Jun 19. Blood. 2017. PMID: 28630122 Free PMC article.
References
-
- Balmer, P., and R. Borrow. 2004. Serologic correlates of protection for evaluating the response to meningococcal vaccines. Expert Rev. Vaccines 377-87. - PubMed
-
- Bjune, G., J. K. Gronnesby, E. A. Hoiby, O. Closs, and H. Nokleby. 1991. Results of an efficacy trial with an outer membrane vesicle vaccine against systemic serogroup B meningococcal disease in Norway. NIPH Ann. 14125-130. - PubMed
-
- Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 232222-2227. - PubMed
-
- Borrow, R., G. M. Carlone, N. Rosenstein, M. Blake, I. Feavers, D. Martin, W. Zollinger, J. Robbins, I. Aaberge, D. M. Granoff, E. Miller, B. Plikaytis, L. van Alphen, J. Poolman, R. Rappuoli, L. Danzig, J. Hackell, B. Danve, M. Caulfield, S. Lambert, and D. Stephens. 2006. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 245093-5107. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

