Distinct classes of red/far-red photochemistry within the phytochrome superfamily
- PMID: 19339496
- PMCID: PMC2669357
- DOI: 10.1073/pnas.0902370106
Distinct classes of red/far-red photochemistry within the phytochrome superfamily
Abstract
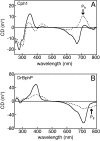
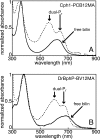
Phytochromes are a widespread family of photosensory proteins first discovered in plants, which measure the ratio of red to far-red light to control many aspects of growth and development. Phytochromes interconvert between red-absorbing P(r) and far-red-absorbing P(fr) states via photoisomerization of a covalently-bound linear tetrapyrrole (bilin) chromophore located in a conserved photosensory core. From recent crystal structures of this core region, it has been inferred that the chromophore structures of P(r) and P(fr) are conserved in most phytochromes. Using circular dichroism spectroscopy and ab initio calculations, we establish that the P(fr) states of the biliverdin-containing bacteriophytochromes DrBphP and PaBphP are structurally dissimilar from those of the phytobilin-containing cyanobacterial phytochrome Cph1. This conclusion is further supported by chromophore substitution experiments using semisynthetic bilin monoamides, which indicate that the propionate side chains perform different functional roles in the 2 classes of phytochromes. We propose that different directions of bilin D-ring rotation account for these distinct classes of red/far-red photochemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gärtner W, Braslavsky SE. The phytochromes: Spectroscopy and function. In: Batschauer A, editor. Photoreceptors and Light Signaling, Comprehensive Series in Photochemical and Photobiological Sciences. Vol 3. Cambridge, UK: Royal Society of Chemistry; 2003. pp. 136–180.
-
- Karniol B, Vierstra RD. Structure, function, and evolution of microbial phytochromes. In: Schäfer E, Nagy F, editors. Photomorphogenesis in Plants and Bacteria: Function and Signal Transduction Mechanisms. 3rd Ed. Dordrecht, The Netherlands: Springer; 2005. pp. 65–98.
-
- Montgomery BL, Lagarias JC. Phytochrome ancestry: Sensors of bilins and light. Trends Plant Sci. 2002;7:357–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

