VTC4 is a bifunctional enzyme that affects myoinositol and ascorbate biosynthesis in plants
- PMID: 19339506
- PMCID: PMC2689953
- DOI: 10.1104/pp.108.135129
VTC4 is a bifunctional enzyme that affects myoinositol and ascorbate biosynthesis in plants
Abstract
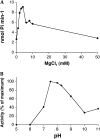
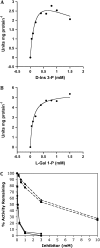
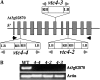
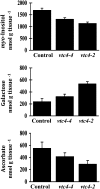
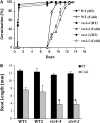
Myoinositol synthesis and catabolism are crucial in many multiceullar eukaryotes for the production of phosphatidylinositol signaling molecules, glycerophosphoinositide membrane anchors, cell wall pectic noncellulosic polysaccharides, and several other molecules including ascorbate. Myoinositol monophosphatase (IMP) is a major enzyme required for the synthesis of myoinositol and the breakdown of myoinositol (1,4,5)trisphosphate, a potent second messenger involved in many biological activities. It has been shown that the VTC4 enzyme from kiwifruit (Actinidia deliciosa) has similarity to IMP and can hydrolyze l-galactose 1-phosphate (l-Gal 1-P), suggesting that this enzyme may be bifunctional and linked with two potential pathways of plant ascorbate synthesis. We describe here the kinetic comparison of the Arabidopsis (Arabidopsis thaliana) recombinant VTC4 with d-myoinositol 3-phosphate (d-Ins 3-P) and l-Gal 1-P. Purified VTC4 has only a small difference in the V(max)/K(m) for l-Gal 1-P as compared with d-Ins 3-P and can utilize other related substrates. Inhibition by either Ca(2+) or Li(+), known to disrupt cell signaling, was the same with both l-Gal 1-P and d-Ins 3-P. To determine whether the VTC4 gene impacts myoinositol synthesis in Arabidopsis, we isolated T-DNA knockout lines of VTC4 that exhibit small perturbations in abscisic acid, salt, and cold responses. Analysis of metabolite levels in vtc4 mutants showed that less myoinositol and ascorbate accumulate in these mutants. Therefore, VTC4 is a bifunctional enzyme that impacts both myoinositol and ascorbate synthesis pathways.
Figures
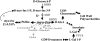


References
-
- Alcazar-Roman AR, Wente SR (2007) Inositol polyphosphates: a new frontier for regulating gene expression. Chromosoma 117 1–13 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 - PubMed
-
- Boss WF, Davis AJ, Im YJ, Galvao RM, Perera IY (2006) Phosphoinositide metabolism: towards an understanding of subcellular signaling. Subcell Biochem 39 181–205 - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

