Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals
- PMID: 19339593
- PMCID: PMC6665392
- DOI: 10.1523/JNEUROSCI.4363-08.2009
Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals
Abstract
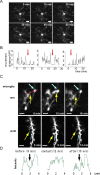
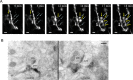
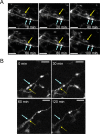
Recent studies have identified the important contribution of glial cells to the plasticity of neuronal circuits. Resting microglia, the primary immune effector cells in the brain, dynamically extend and retract their processes as if actively surveying the microenvironment. However, just what is being sampled by these resting microglial processes has not been demonstrated in vivo, and the nature and function of any interactions between microglia and neuronal circuits is incompletely understood. Using in vivo two-photon imaging of fluorescent-labeled neurons and microglia, we demonstrate that the resting microglial processes make brief (approximately 5 min) and direct contacts with neuronal synapses at a frequency of about once per hour. These contacts are activity-dependent, being reduced in frequency by reductions in neuronal activity. After transient cerebral ischemia, the duration of these microglia-synapse contacts are markedly prolonged (approximately 1 h) and are frequently followed by the disappearance of the presynaptic bouton. Our results demonstrate that at least part of the dynamic motility of resting microglial processes in vivo is directed toward synapses and propose that microglia vigilantly monitor and respond to the functional status of synapses. Furthermore, the striking finding that some synapses in the ischemic areas disappear after prolonged microglial contact suggests microglia contribute to the subsequent increased turnover of synaptic connections. Further understanding of the mechanisms involved in the microglial detection of the functional state of synapses, and of their role in remodeling neuronal circuits disrupted by ischemia, may lead to novel therapies for treating brain injury that target microglia.
Figures

References
-
- Bessis A, Béchade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. - PubMed
-
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. - PubMed
-
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85:145–157. - PubMed
-
- Bruce-Keller AJ. Microglial-neuronal interactions in synaptic damage and recovery. J Neurosci Res. 1999;58:191–201. - PubMed
-
- Colman H, Nabekura J, Lichtman JW. Alterations in synaptic strength preceding axon withdrawal. Science. 1997;275:356–361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
