BARHL2 differentially regulates the development of retinal amacrine and ganglion neurons
- PMID: 19339595
- PMCID: PMC2756297
- DOI: 10.1523/JNEUROSCI.5237-08.2009
BARHL2 differentially regulates the development of retinal amacrine and ganglion neurons
Abstract
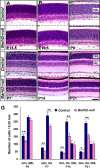
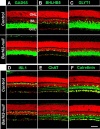
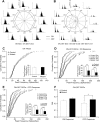
Through transcriptional regulations, the BarH family of homeodomain proteins play essential roles in cell fate specification, cell differentiation, migration, and survival. Barhl2, a member of the Barh gene family, is expressed in retinal ganglion cells (RGCs), amacrine cells (ACs), and horizontal cells. Here, to investigate the role of Barhl2 in retinal development, Barhl2-deficient mice were generated. Analysis of AC subtypes in Barhl2-deficient retinas suggests that Barhl2 plays a critical role in AC subtype determination. A significant reduction of glycinergic and GABAergic ACs with a substantial increase in the number of cholinergic ACs was observed in Barhl2-null retinas. Barhl2 is also critical for the development of a normal complement of RGCs. Barhl2 deficiency resulted in a 35% increase in RGCs undergoing apoptosis during development. Genetic analysis revealed that Barhl2 functions downstream of the Atoh7-Pou4f3 regulatory pathway and regulates the maturation and/or survival of RGCs. Thus, BARHL2 appears to have numerous roles in retinal development, including regulating neuronal subtype specification, differentiation, and survival.
Figures







References
-
- Auerbach W, Dunmore JH, Fairchild-Huntress V, Fang Q, Auerbach AB, Huszar D, Joyner AL. Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines. Biotechniques. 2000;29:1024–1028. 1030, 1032. - PubMed
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. - PMC - PubMed
-
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. - PubMed
-
- Bramblett DE, Pennesi ME, Wu SM, Tsai MJ. The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron. 2004;43:779–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
