Cholinergic deafferentation of the neocortex using 192 IgG-saporin impairs feature binding in rats
- PMID: 19339607
- PMCID: PMC6665388
- DOI: 10.1523/JNEUROSCI.0654-09.2009
Cholinergic deafferentation of the neocortex using 192 IgG-saporin impairs feature binding in rats
Abstract
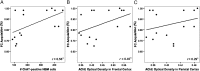
The binding problem refers to the fundamental challenge of the CNS to integrate sensory information registered by distinct brain regions to form a unified neural representation of a stimulus. Although the human cognitive literature has established that attentional processes in frontoparietal cortices support feature binding, the neurochemical and specific downstream neuroanatomical contributions to feature binding remain unknown. Using systemic pharmacology in rats, it has been shown that the neuromodulator acetylcholine is essential for feature binding at encoding, but the neural source of such critical cholinergic neurotransmission has yet to be identified. Cholinergic efferents from the nucleus basalis magnocellularis (NBM) of the basal forebrain provide the majority of the cholinergic input to the neocortex. Accordingly, it was hypothesized that the NBM is the neural source that provides the critical neuromodulatory support for feature binding. To test this hypothesis, rats received bilateral 192 IgG-saporin lesions of the NBM, and their feature binding performance was tested using a forced-choice digging paradigm. Relative to sham-lesioned rats, NBM-lesioned rats were significantly impaired at acquiring a crossmodal feature conjunction (FC) stimulus set that required feature binding, whereas their ability to retrieve an FC stimulus set and to acquire two crossmodal feature singleton stimulus sets, one of greater difficulty than the other but neither requiring feature binding, remained intact. These behavioral findings, along with histological analyses demonstrating positive relationships between feature-binding acquisition and markers of cholinergic activity in frontoparietal regions, reveal the importance of neocortical cholinergic input from the NBM to feature binding at encoding.
Figures







References
-
- Aigner TG, Walker DL, Mishkin M. Comparison of the effects of scopolamine administered before and after acquisition in a test of visual recognition memory in monkeys. Behav Neural Biol. 1991;55:61–67. - PubMed
-
- Anagnostaras SG, Maren S, Fanselow MS. Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem. 1995;64:191–194. - PubMed
-
- Atri A, Sherman S, Norman KA, Kirchhoff BA, Nicolas MM, Greicius MD, Cramer SC, Breiter HC, Hasselmo ME, Stern CE. Blockade of central cholinergic receptors impairs new learning and increases proactive interference in a word paired-associate memory task. Behav Neurosci. 2004;118:223–236. - PubMed
-
- Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci. 1995;109:714–722. - PubMed
-
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
