Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice
- PMID: 19339610
- PMCID: PMC3437665
- DOI: 10.1523/JNEUROSCI.5256-08.2009
Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice
Abstract
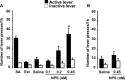
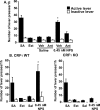
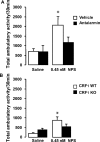
Neuropeptide S (NPS) is a recently discovered neuropeptide that increases arousal and wakefulness while decreasing anxiety-like behavior. Here, we used a self-administration paradigm to demonstrate that intracerebroventricular infusion of NPS reinstates extinguished cocaine-seeking behavior in a dose-dependent manner in mice. The highest dose of NPS (0.45 nM) increased active lever pressing in the absence of cocaine to levels that were equivalent to those observed during self-administration. In addition, we examined the role of the corticotropin-releasing factor receptor 1 (CRF(1)) in this behavior as well as locomotor stimulation and anxiolysis. CRF(1) knock-out mice did not respond to either the locomotor stimulant or cocaine reinstatement effects of NPS, but still responded to its anxiolytic effect. The CRF(1) antagonist antalarmin also blocked the increase in active lever responding in the reinstatement model and the locomotor activating properties of NPS without affecting its anxiolytic actions. Our results suggest that NPS receptors may be an important target for drug abuse research and treatment and that CRF(1) mediates the cocaine-seeking and locomotor stimulant effects of NPS, but not its effects on anxiety-like behavior.
Figures

Similar articles
-
Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2.J Neurosci. 2011 Aug 3;31(31):11396-403. doi: 10.1523/JNEUROSCI.1393-11.2011. J Neurosci. 2011. PMID: 21813699 Free PMC article.
-
Beta-2 adrenergic receptors mediate stress-evoked reinstatement of cocaine-induced conditioned place preference and increases in CRF mRNA in the bed nucleus of the stria terminalis in mice.Psychopharmacology (Berl). 2014 Oct;231(20):3953-63. doi: 10.1007/s00213-014-3535-0. Epub 2014 Apr 3. Psychopharmacology (Berl). 2014. PMID: 24696080 Free PMC article.
-
Locomotor activity and cocaine-seeking behavior during acquisition and reinstatement of operant self-administration behavior in rats.Behav Brain Res. 2005 May 28;160(2):250-9. doi: 10.1016/j.bbr.2004.12.005. Epub 2005 Jan 12. Behav Brain Res. 2005. PMID: 15863221
-
A Role for Neuropeptide S in Alcohol and Cocaine Seeking.Pharmaceuticals (Basel). 2022 Jun 27;15(7):800. doi: 10.3390/ph15070800. Pharmaceuticals (Basel). 2022. PMID: 35890099 Free PMC article. Review.
-
Can Neuropeptide S Be an Indicator for Assessing Anxiety in Psychiatric Disorders?Front Public Health. 2022 Apr 26;10:872430. doi: 10.3389/fpubh.2022.872430. eCollection 2022. Front Public Health. 2022. PMID: 35558538 Free PMC article. Review.
Cited by
-
Characterization of Highper, an ENU-induced mouse mutant with abnormal psychostimulant and stress responses.Psychopharmacology (Berl). 2013 Jan;225(2):407-19. doi: 10.1007/s00213-012-2827-5. Epub 2012 Sep 5. Psychopharmacology (Berl). 2013. PMID: 22948668 Free PMC article.
-
Blockade of adenosine A2A receptor counteracts neuropeptide-S-induced hyperlocomotion in mice.Naunyn Schmiedebergs Arch Pharmacol. 2010 Feb;381(2):153-60. doi: 10.1007/s00210-009-0480-2. Epub 2009 Dec 19. Naunyn Schmiedebergs Arch Pharmacol. 2010. PMID: 20020280
-
Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior.J Neurosci. 2010 Feb 10;30(6):2300-10. doi: 10.1523/JNEUROSCI.5724-09.2010. J Neurosci. 2010. PMID: 20147556 Free PMC article.
-
Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats.Psychopharmacology (Berl). 2016 Aug;233(15-16):2915-24. doi: 10.1007/s00213-016-4333-7. Epub 2016 May 28. Psychopharmacology (Berl). 2016. PMID: 27235017 Free PMC article.
-
Pharmacology, Physiology and Genetics of the Neuropeptide S System.Pharmaceuticals (Basel). 2021 Apr 23;14(5):401. doi: 10.3390/ph14050401. Pharmaceuticals (Basel). 2021. PMID: 33922620 Free PMC article. Review.
References
-
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. - PubMed
-
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. - PubMed
-
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. - PubMed
-
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice. Ed 2. Hoboken, NJ: Wiley-Interscience; 2007. What's wrong with my mouse?
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
