Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus
- PMID: 19339615
- PMCID: PMC4487620
- DOI: 10.1523/JNEUROSCI.0208-09.2009
Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus
Abstract
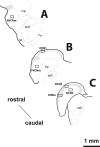
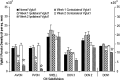
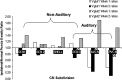
Integration of multimodal information is essential for understanding complex environments. In the auditory system, multisensory integration first occurs in the cochlear nucleus (CN), where auditory nerve and somatosensory pathways converge (Shore, 2005). A unique feature of multisensory neurons is their propensity to receive cross-modal compensation after deafening. Based on our findings that the vesicular glutamate transporters, VGLUT1 and VGLUT2, are differentially associated with auditory nerve and somatosensory inputs to the CN, respectively (Zhou et al., 2007), we examined their relative distributions after unilateral deafening. After unilateral intracochlear injections of kanamycin (1 and 2 weeks), VGLUT1 immunoreactivity (ir) in the magnocellular CN ipsilateral to the cochlear damage was significantly decreased, whereas VGLUT2-ir in regions that receive nonauditory input was significantly increased 2 weeks after deafening. The pathway-specific amplification of VGLUT2 expression in the CN suggests that, in compensatory response to deafening, the nonauditory influence on CN is significantly enhanced. One undesirable consequence of enhanced glutamatergic inputs could be the increased spontaneous rates in CN neurons that occur after hearing loss and that have been proposed as correlates of the phantom auditory sensations commonly called tinnitus.
Figures



References
-
- Alibardi L. Mossy fibers in granule cell areas of the rat dorsal cochlear nucleus from intrinsic and extrinsic origin innervate unipolar brush cell glomeruli. J Submicrosc Cytol Pathol. 2004;36:193–210. - PubMed
-
- Babalian AL. Synaptic influences of pontine nuclei on cochlear nucleus cells. Exp Brain Res. 2005;167:451–457. - PubMed
-
- Batzri-Izraeli R, Kelly JB, Glendenning KK, Masterton RB, Wollberg Z. Auditory cortex of the long-eared hedgehog (Hemiechinus auritus). I. Boundaries and frequency representation. Brain Behav Evol. 1990;36:237–248. - PubMed
-
- Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J Neurosci Res. 2007;85:1489–1498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
