A SnoN-Ccd1 pathway promotes axonal morphogenesis in the mammalian brain
- PMID: 19339625
- PMCID: PMC2853192
- DOI: 10.1523/JNEUROSCI.0126-09.2009
A SnoN-Ccd1 pathway promotes axonal morphogenesis in the mammalian brain
Abstract
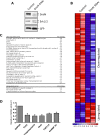
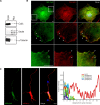
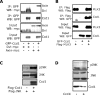
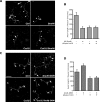
The transcriptional corepressor SnoN is a critical regulator of axonal morphogenesis, but how SnoN drives axonal growth is unknown. Here, we report that gene-profiling analyses in cerebellar granule neurons reveal that the large majority of genes altered upon SnoN knockdown are surprisingly downregulated, suggesting that SnoN may activate transcription in neurons. Accordingly, we find that the transcriptional coactivator p300 interacts with SnoN, and p300 plays a critical role in SnoN-induced axon growth. We also identify the gene encoding the signaling scaffold protein Ccd1 as a critical target of SnoN in neurons. Ccd1 localizes to the actin cytoskeleton, is enriched at axon terminals in neurons, and activates the axon growth-promoting kinase JNK (c-Jun N-terminal protein kinase). Knockdown of Ccd1 in neurons reduces axonal length and suppresses the ability of SnoN to promote axonal growth. Importantly, Ccd1 knockdown in rat pups profoundly impairs the formation of granule neuron parallel fiber axons in the rat cerebellar cortex in vivo. These findings define a novel SnoN-Ccd1 link that promotes axonal growth in the mammalian brain, with important implications for axonal development and regeneration.
Figures




Similar articles
-
Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN.Neuron. 2006 May 4;50(3):389-400. doi: 10.1016/j.neuron.2006.03.034. Neuron. 2006. PMID: 16675394
-
TGFbeta-Smad2 signaling regulates the Cdh1-APC/SnoN pathway of axonal morphogenesis.J Neurosci. 2008 Feb 20;28(8):1961-9. doi: 10.1523/JNEUROSCI.3061-07.2008. J Neurosci. 2008. PMID: 18287512 Free PMC article.
-
Axon formation in neocortical neurons depends on stage-specific regulation of microtubule stability by the dual leucine zipper kinase-c-Jun N-terminal kinase pathway.J Neurosci. 2011 Apr 27;31(17):6468-80. doi: 10.1523/JNEUROSCI.5038-10.2011. J Neurosci. 2011. PMID: 21525288 Free PMC article.
-
SnoN signaling in proliferating cells and postmitotic neurons.FEBS Lett. 2012 Jul 4;586(14):1977-83. doi: 10.1016/j.febslet.2012.02.048. Epub 2012 Mar 8. FEBS Lett. 2012. PMID: 22710173 Free PMC article. Review.
-
SnoN in regulation of embryonic development and tissue morphogenesis.FEBS Lett. 2012 Jul 4;586(14):1971-6. doi: 10.1016/j.febslet.2012.03.005. Epub 2012 Mar 10. FEBS Lett. 2012. PMID: 22710172 Free PMC article. Review.
Cited by
-
The Sno oncogene antagonizes Wingless signaling during wing development in Drosophila.PLoS One. 2010 Jul 16;5(7):e11619. doi: 10.1371/journal.pone.0011619. PLoS One. 2010. PMID: 20661280 Free PMC article.
-
Extracellular lactate improves neurogenesis by modulating H3K9 lactylation and SnoN expression under hypoxic conditions.Stem Cell Res Ther. 2025 Aug 27;16(1):462. doi: 10.1186/s13287-025-04571-4. Stem Cell Res Ther. 2025. PMID: 40866997 Free PMC article.
-
DIXDC1 contributes to psychiatric susceptibility by regulating dendritic spine and glutamatergic synapse density via GSK3 and Wnt/β-catenin signaling.Mol Psychiatry. 2018 Feb;23(2):467-475. doi: 10.1038/mp.2016.184. Epub 2016 Oct 18. Mol Psychiatry. 2018. PMID: 27752079 Free PMC article.
-
Transcriptional cofactors Ski and SnoN are major regulators of the TGF-β/Smad signaling pathway in health and disease.Signal Transduct Target Ther. 2018 Jun 8;3:15. doi: 10.1038/s41392-018-0015-8. eCollection 2018. Signal Transduct Target Ther. 2018. PMID: 29892481 Free PMC article.
-
Regulation of Cdh1-APC function in axon growth by Cdh1 phosphorylation.J Neurosci. 2009 Apr 1;29(13):4322-7. doi: 10.1523/JNEUROSCI.5329-08.2009. J Neurosci. 2009. PMID: 19339626 Free PMC article.
References
-
- Altman J, Bayer S. Development of the cerebellar system: in relation to its evolution, structure, and functions. New York: CRC; 1997.
-
- Butler SJ, Tear G. Getting axons onto the right path: the role of transcription factors in axon guidance. Development. 2007;134:439–448. - PubMed
-
- Chang E, Goldberg H. Requirements for transforming growth factor-beta regulation of the pro-alpha 2(I) collagen and plasminogen activator inhibitor-1 promoters. J Biol Chem. 1995;270:4473–4477. - PubMed
-
- Gaudilliere B, Shi Y, Bonni A. RNA interference reveals a requirement for myocyte enhancer factor 2A in activity-dependent neuronal survival. J Biol Chem. 2002;277:46442–46446. - PubMed
-
- Gaudillière B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
