Manipulating actin dynamics affects human in vitro decidualization
- PMID: 19339710
- PMCID: PMC3093994
- DOI: 10.1095/biolreprod.108.074666
Manipulating actin dynamics affects human in vitro decidualization
Abstract
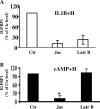
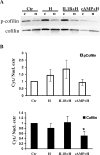
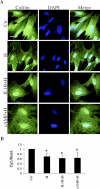
The differentiation of uterine stromal fibroblasts into decidual cells is critical for establishing pregnancy. This process, called decidualization, requires the reorganization of the actin cytoskeleton, which mainly depends on actin dynamics and the phosphorylation status of the myosin light chain. We manipulated actin dynamics with jasplakinolide (100 nM) and latrunculin B (1 microM), both of which significantly inhibited the synthesis of decidualization markers induced by 6 days of treatment with embryo-mimicking stimulus interleukin 1beta (IL1B) and steroid hormones (SHs; 17beta-estradiol and medroxyprogesterone acetate) in the human uterine fibroblast (HuF) in vitro model. However, only jasplakinolide had long-lasting effects on the G-actin:F-actin ratio and prevented decidualization induced by the artificial stimulus cAMP (and SHs). Actin-binding protein cofilin mainly colocalized with G-actin in the nucleus as well as the cytoplasm. Only some spots of colocalization between cofilin and F-actin were detected in the cytoplasm. Brief extraction of cytosolic proteins from living cells revealed that in cells treated with IL1B or cAMP (and SHs) for 6 days, cofilin was mainly detected in the nucleus. The translocation of cofilin from cytosol to nucleus was also detected in HuFs treated for 12 days with SHs, IL1B and SHs, and cAMP and SHs. The same significant translocation was confirmed in primary baboon stromal uterine fibroblasts. We conclude that changes in actin dynamics, particularly the stabilization of F-actin, have a significant negative impact on decidualization, and the translocation of cofilin to the nucleus is a key feature of this process in the primate.
Figures




Similar articles
-
Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human.Biol Reprod. 1998 Jul;59(1):160-8. doi: 10.1095/biolreprod59.1.160. Biol Reprod. 1998. PMID: 9675007
-
Increased phosphorylation of myosin light chain prevents in vitro decidualization.Endocrinology. 2007 Jul;148(7):3176-84. doi: 10.1210/en.2006-1673. Epub 2007 Apr 5. Endocrinology. 2007. PMID: 17412815
-
Human transcriptional coactivator with PDZ-binding motif (TAZ) is downregulated during decidualization.Biol Reprod. 2010 Jun;82(6):1112-8. doi: 10.1095/biolreprod.109.081844. Epub 2010 Feb 17. Biol Reprod. 2010. PMID: 20164440 Free PMC article.
-
IL-1beta during in vitro decidualization in primate.J Reprod Immunol. 2002 May-Jun;55(1-2):35-47. doi: 10.1016/s0165-0378(01)00141-3. J Reprod Immunol. 2002. PMID: 12062820 Review.
-
Blastocyst invasion and the stromal response in primates.Hum Reprod. 1999 Dec;14 Suppl 2:45-55. doi: 10.1093/humrep/14.suppl_2.45. Hum Reprod. 1999. PMID: 10690800 Review.
Cited by
-
ATOH8 Expression Is Regulated by BMP2 and Plays a Key Role in Human Endometrial Stromal Cell Decidualization.Endocrinology. 2023 Nov 20;165(1):bqad188. doi: 10.1210/endocr/bqad188. Endocrinology. 2023. PMID: 38060684 Free PMC article.
-
Three-dimensional microengineered vascularised endometrium-on-a-chip.Hum Reprod. 2021 Sep 18;36(10):2720-2731. doi: 10.1093/humrep/deab186. Hum Reprod. 2021. PMID: 34363466 Free PMC article.
-
Immune Tolerance of Embryo Implantation and Pregnancy: The Role of Human Decidual Stromal Cell- and Embryonic-Derived Extracellular Vesicles.Int J Mol Sci. 2022 Nov 2;23(21):13382. doi: 10.3390/ijms232113382. Int J Mol Sci. 2022. PMID: 36362169 Free PMC article. Review.
-
Flow-enhanced priming of hESCs through H2B acetylation and chromatin decondensation.Stem Cell Res Ther. 2019 Nov 27;10(1):349. doi: 10.1186/s13287-019-1454-z. Stem Cell Res Ther. 2019. PMID: 31775893 Free PMC article.
-
Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology.Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):E8468-E8477. doi: 10.1073/pnas.1706546114. Epub 2017 Sep 18. Proc Natl Acad Sci U S A. 2017. PMID: 28923940 Free PMC article.
References
-
- Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, Brudney A, Knight O, Fazleabas AT. In vivo infusion of interleukin-1β and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology 2005; 146: 4097 4104 - PubMed
-
- Kim JJ, Jaffe RC, Fazleabas AT. Insulin-like growth factor binding protein-1 expression in baboon endometrial stromal cells: regulation by filamentous actin and requirement for de novo protein synthesis. Endocrinology 1999; 140: 997 1004 - PubMed
-
- Ihnatovych I, Hu W, Martin JL, Fazleabas AT, de Lanerolle P, Strakova Z. Increased phosphorylation of myosin light chain prevents in vitro decidualization. Endocrinology 2007; 148: 3176 3184 - PubMed
-
- Paavilainen VO, Bertling E, Falck S, Lappalainen P. Regulation of cytoskeletal dynamics by actin-monomer-binding proteins. Trends Cell Biol 2004; 14: 386 394 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

