Bipolar disorder: from genes to behavior pathways
- PMID: 19339764
- PMCID: PMC2662565
- DOI: 10.1172/JCI37703
Bipolar disorder: from genes to behavior pathways
Abstract
Bipolar disorder (BPD) is a devastating illness that is characterized by recurrent episodes of mania and depression. In addition to these cyclic episodes, individuals with BPD exhibit changes in psychovegetative function, cognitive performance, and general health and well being. In this article we draw from neuroimaging findings in humans, postmortem data, and human genetic and pharmacological studies as well as data from animal models of behavior to discuss the neurobiology of BPD. We conclude with a synthesis of where the field stands and with suggestions and strategies for future areas of study to further increase our conceptual understanding of this complex illness.
Figures
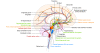
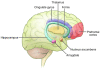
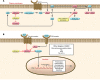
References
-
- Akiskal H.S., et al. Re-evaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. . J. Affect. Disord. 2000;59(Suppl. 1):S5–S30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

