Chemokine receptors and other G protein-coupled receptors
- PMID: 19339946
- PMCID: PMC2771364
- DOI: 10.1097/COH.0b013e3283223d8d
Chemokine receptors and other G protein-coupled receptors
Abstract
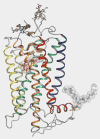
Purpose of review: Class A G protein-coupled receptors (GPCRs), including the chemokine receptors, CCR5 and CXCR4, share a seven transmembrane-spanning alpha-helix architecture that accommodates signal propagation from across biological membranes. CXCR4 and CCR5 are utilized as co-receptors during HIV viral entry and, therefore, crystal structures of GPCRs aid in the understanding of their function in viral entry.
Recent findings: Recent progress in structure determination of class A GPCRs, which include vertebrate and invertebrate rhodopsin as well as adrenergic and adenosine receptors, provides molecular templates for how this diverse group of transmembrane receptors functions. Each of these GPCRs differs in how specific ligands bind to the transmembrane core, underscoring that additional structures of GPCRs from other subfamilies are needed to facilitate rational drug design. More recent studies also indicate a need to consider the more complex character of GPCRs, such as their oligomerization and dynamics.
Summary: Recently, the atomic structures of invertebrate rhodopsin, beta1-adrenergic and beta2-adrenergic receptors and the A(2A)-adenosine receptor have been solved via X-ray crystallography. The impact that these structures have on the biochemistry of viral entry and signal transduction is discussed. Because the chemokine receptors have proven refractory to structural studies thus far, further structural study of the chemokine receptors will be essential to understanding ligand binding, activation and function as co-receptors during viral entry.
Figures




References
-
- Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. - PubMed
-
- Kroeze WK, Sheffler DJ, Roth BL. G-protein-coupled receptors at a glance. J Cell Sci. 2003;116:4867–4869. - PubMed
-
- Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. - PubMed
-
- Filipek S, Krzysko KA, Fotiadis D, Liang Y, Saperstein DA, Engel A, Palczewski K. A concept for G protein activation by G protein-coupled receptor dimers: the transducin/rhodopsin interface. Photochem Photobiol Sci. 2004;3:628–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

