Structural basis for leucine-rich nuclear export signal recognition by CRM1
- PMID: 19339969
- PMCID: PMC3437623
- DOI: 10.1038/nature07975
Structural basis for leucine-rich nuclear export signal recognition by CRM1
Erratum in
- Nature. 2009 Sep 24;461(7263):550
Abstract
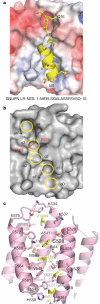
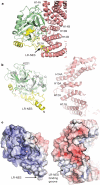
CRM1 (also known as XPO1 and exportin 1) mediates nuclear export of hundreds of proteins through the recognition of the leucine-rich nuclear export signal (LR-NES). Here we present the 2.9 A structure of CRM1 bound to snurportin 1 (SNUPN). Snurportin 1 binds CRM1 in a bipartite manner by means of an amino-terminal LR-NES and its nucleotide-binding domain. The LR-NES is a combined alpha-helical-extended structure that occupies a hydrophobic groove between two CRM1 outer helices. The LR-NES interface explains the consensus hydrophobic pattern, preference for intervening electronegative residues and inhibition by leptomycin B. The second nuclear export signal epitope is a basic surface on the snurportin 1 nucleotide-binding domain, which binds an acidic patch on CRM1 adjacent to the LR-NES site. Multipartite recognition of individually weak nuclear export signal epitopes may be common to CRM1 substrates, enhancing CRM1 binding beyond the generally low affinity LR-NES. Similar energetic construction is also used in multipartite nuclear localization signals to provide broad substrate specificity and rapid evolution in nuclear transport.
Figures


References
-
- Tran EJ, Bolger TA, Wente SR. SnapShot: nuclear transport. Cell. 2007;131:420. - PubMed
-
- Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–451. - PubMed
-
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999;15:607–660. - PubMed
-
- Conti E, Izaurralde E. Nucleocytoplasmic transport enters the atomic age. Curr. Opin. Cell Biol. 2001;13:310–319. - PubMed
-
- Dingwall C, Sharnick SV, Laskey RA. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982;30:449–458. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

