The neuro-symphony of stress
- PMID: 19339973
- PMCID: PMC2844123
- DOI: 10.1038/nrn2632
The neuro-symphony of stress
Abstract
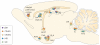
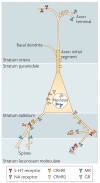
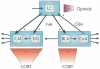
The impact of stress on brain function is increasingly recognized. Various substances are released in response to stress and can influence distinct neuronal circuits, but the functional advantages of having such a diversity of stress mediators remain unclear. Individual neurotransmitter, neuropeptide and steroid stress mediators have specific spatial and temporal niches, but these niches also overlap. In addition, the effects of individual mediators on neuronal function and plasticity are integrated, and emerging evidence suggests that there is crosstalk between them. Together, this results in the stress instruments producing an orchestrated 'symphony' that enables fine-tuned responses to diverse challenges.
Figures

References
-
- De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Rev. Neurosci. 2005;6:463–475. - PubMed
-
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. - PubMed
-
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

