Ion channels versus ion pumps: the principal difference, in principle
- PMID: 19339978
- PMCID: PMC2742554
- DOI: 10.1038/nrm2668
Ion channels versus ion pumps: the principal difference, in principle
Abstract
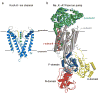
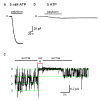
The incessant traffic of ions across cell membranes is controlled by two kinds of border guards: ion channels and ion pumps. Open channels let selected ions diffuse rapidly down electrical and concentration gradients, whereas ion pumps labour tirelessly to maintain the gradients by consuming energy to slowly move ions thermodynamically uphill. Because of the diametrically opposed tasks and the divergent speeds of channels and pumps, they have traditionally been viewed as completely different entities, as alike as chalk and cheese. But new structural and mechanistic information about both of these classes of molecular machines challenges this comfortable separation and forces its re-evaluation.
Figures



References
-
- Hille B. Ion channels of excitable membranes. Vol. 814. Sinauer; Sunderland, MA: 2001.
-
- Sakmann B, Neher E. Single-Channel Recording. Plenum; New York: 1995.
-
- Läuger P. A channel mechanism for electrogenic ion pumps. Biochim Biophys Acta. 1979;552:143–161. - PubMed
-
- Patlak CS. Contributions to the theory of active transport. II. The gate-type non-carrier mechanism and generalization concerning tracer glow efficiency, and measurement of energy expenditure. Bull Math Biophys. 1957;19:209–235.
-
- Vidaver GA. Inhibition of parallel flux and augmentation of counter flux shown by transport models not involving a mobile carrier. J Theor Biol. 1966;10:301–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

