Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes
- PMID: 19339990
- PMCID: PMC2683056
- DOI: 10.1038/emboj.2009.82
Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes
Abstract
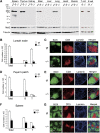
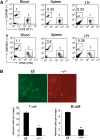
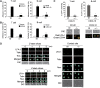
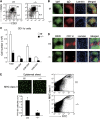
The regulation of lymphocyte adhesion and migration plays crucial roles in lymphocyte trafficking during immunosurveillance. However, our understanding of the intracellular signalling that regulates these processes is still limited. Here, we show that the Ste20-like kinase Mst1 plays crucial roles in lymphocyte trafficking in vivo. Mst1(-/-) lymphocytes exhibited an impairment of firm adhesion to high endothelial venules, resulting in an inefficient homing capacity. In vitro lymphocyte adhesion cascade assays under physiological shear flow revealed that the stopping time of Mst1(-/-) lymphocytes on endothelium was markedly reduced, whereas their L-selectin-dependent rolling/tethering and transition to LFA-1-mediated arrest were not affected. Mst1(-/-) lymphocytes were also defective in the stabilization of adhesion through alpha4 integrins. Consequently, Mst1(-/-) mice had hypotrophic peripheral lymphoid tissues and reduced marginal zone B cells and dendritic cells in the spleen, and defective emigration of single positive thymocytes. Furthermore, Mst1(-/-) lymphocytes had impaired motility over lymph node-derived stromal cells and within lymph nodes. Thus, our data indicate that Mst1 is a key enzyme involved in lymphocyte entry and interstitial migration.
Figures



Similar articles
-
The Mst1 Kinase Is Required for Follicular B Cell Homing and B-1 B Cell Development.Front Immunol. 2018 Oct 17;9:2393. doi: 10.3389/fimmu.2018.02393. eCollection 2018. Front Immunol. 2018. PMID: 30386341 Free PMC article.
-
Rab13 acts downstream of the kinase Mst1 to deliver the integrin LFA-1 to the cell surface for lymphocyte trafficking.Sci Signal. 2014 Jul 29;7(336):ra72. doi: 10.1126/scisignal.2005199. Sci Signal. 2014. PMID: 25074980
-
A cell-intrinsic role for Mst1 in regulating thymocyte egress.J Immunol. 2009 Sep 15;183(6):3865-72. doi: 10.4049/jimmunol.0900678. Epub 2009 Aug 19. J Immunol. 2009. PMID: 19692642
-
In situ analysis of lymphocyte migration to lymph nodes.Cell Adhes Commun. 1998;6(2-3):85-96. doi: 10.3109/15419069809004463. Cell Adhes Commun. 1998. PMID: 9823458 Review.
-
Lymphocyte cell motility: the twisting, turning tale of phosphoinositide 3-kinase.Biochem Soc Trans. 2007 Nov;35(Pt 5):1109-13. doi: 10.1042/BST0351109. Biochem Soc Trans. 2007. PMID: 17956290 Free PMC article. Review.
Cited by
-
Tumor suppressor Hippo/MST1 kinase mediates chemotaxis by regulating spreading and adhesion.Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13632-7. doi: 10.1073/pnas.1211304109. Epub 2012 Jul 30. Proc Natl Acad Sci U S A. 2012. PMID: 22847424 Free PMC article.
-
Germinal center kinases in immune regulation.Cell Mol Immunol. 2012 Nov;9(6):439-45. doi: 10.1038/cmi.2012.30. Epub 2012 Sep 10. Cell Mol Immunol. 2012. PMID: 22960604 Free PMC article. Review.
-
TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection.Nat Commun. 2021 Jun 10;12(1):3519. doi: 10.1038/s41467-021-23683-y. Nat Commun. 2021. PMID: 34112781 Free PMC article.
-
The complex entanglement of Hippo-Yap/Taz signaling in tumor immunity.Oncogene. 2019 Apr;38(16):2899-2909. doi: 10.1038/s41388-018-0649-6. Epub 2019 Jan 7. Oncogene. 2019. PMID: 30617303 Free PMC article. Review.
-
The Mst1 Kinase Is Required for Follicular B Cell Homing and B-1 B Cell Development.Front Immunol. 2018 Oct 17;9:2393. doi: 10.3389/fimmu.2018.02393. eCollection 2018. Front Immunol. 2018. PMID: 30386341 Free PMC article.
References
-
- Alon R, Feigelson S (2002) From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol 14: 93–104 - PubMed
-
- Bos JL, de Rooij J, Reedquist KA (2001) Rap1 signaling: adhering to new models. Nat Rev 2: 369–377 - PubMed
-
- Bousso P, Robey E (2003) Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol 4: 579–585 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

