ER stress protects from retinal degeneration
- PMID: 19339992
- PMCID: PMC2683051
- DOI: 10.1038/emboj.2009.76
ER stress protects from retinal degeneration
Abstract
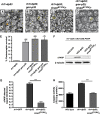
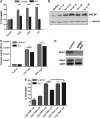
The unfolded protein response (UPR) is a specific cellular process that allows the cell to cope with the overload of unfolded/misfolded proteins in the endoplasmic reticulum (ER). ER stress is commonly associated with degenerative pathologies, but its role in disease progression is still a matter for debate. Here, we found that mutations in the ER-resident chaperone, neither inactivation nor afterpotential A (NinaA), lead to mild ER stress, protecting photoreceptor neurons from various death stimuli in adult Drosophila. In addition, Drosophila S2 cultured cells, when pre-exposed to mild ER stress, are protected from H(2)O(2), cycloheximide- or ultraviolet-induced cell death. We show that a specific ER-mediated signal promotes antioxidant defences and inhibits caspase-dependent cell death. We propose that an immediate consequence of the UPR not only limits the accumulation of misfolded proteins but also protects tissues from harmful exogenous stresses.
Figures
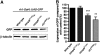

Similar articles
-
Unfolded protein response in a Drosophila model for retinal degeneration.EMBO J. 2007 Jan 10;26(1):242-52. doi: 10.1038/sj.emboj.7601477. Epub 2006 Dec 14. EMBO J. 2007. PMID: 17170705 Free PMC article.
-
Suppression of retinal degeneration in Drosophila by stimulation of ER-associated degradation.Proc Natl Acad Sci U S A. 2009 Oct 6;106(40):17043-8. doi: 10.1073/pnas.0905566106. Epub 2009 Sep 23. Proc Natl Acad Sci U S A. 2009. PMID: 19805114 Free PMC article.
-
Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: effect of an Alzheimer's disease-linked presenilin-1 knock-in mutation.J Biol Chem. 2001 Nov 30;276(48):44736-43. doi: 10.1074/jbc.M104092200. Epub 2001 Sep 26. J Biol Chem. 2001. PMID: 11574534
-
[Involvement of unfolded protein responses in neurodegeneration].Nihon Shinkei Seishin Yakurigaku Zasshi. 2003 Jun;23(3):105-9. Nihon Shinkei Seishin Yakurigaku Zasshi. 2003. PMID: 12884750 Review. Japanese.
-
ER chaperone functions during normal and stress conditions.J Chem Neuroanat. 2004 Sep;28(1-2):51-65. doi: 10.1016/j.jchemneu.2003.08.007. J Chem Neuroanat. 2004. PMID: 15363491 Review.
Cited by
-
Drosophila p53 isoforms differentially regulate apoptosis and apoptosis-induced proliferation.Cell Death Differ. 2013 Jan;20(1):108-16. doi: 10.1038/cdd.2012.100. Epub 2012 Aug 17. Cell Death Differ. 2013. PMID: 22898807 Free PMC article.
-
Protection of retina by mini-αA in NaIO3-induced retinal pigment epithelium degeneration mice.Int J Mol Sci. 2015 Jan 12;16(1):1644-56. doi: 10.3390/ijms16011644. Int J Mol Sci. 2015. PMID: 25588217 Free PMC article.
-
The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy.Prog Retin Eye Res. 2018 Jan;62:1-23. doi: 10.1016/j.preteyeres.2017.10.002. Epub 2017 Oct 16. Prog Retin Eye Res. 2018. PMID: 29042326 Free PMC article. Review.
-
Induction of excessive endoplasmic reticulum stress in the Drosophila male accessory gland results in infertility.PLoS One. 2015 Mar 5;10(3):e0119386. doi: 10.1371/journal.pone.0119386. eCollection 2015. PLoS One. 2015. PMID: 25742606 Free PMC article.
-
Endoplasmic reticulum stress-associated cone photoreceptor degeneration in cyclic nucleotide-gated channel deficiency.J Biol Chem. 2012 May 25;287(22):18018-29. doi: 10.1074/jbc.M112.342220. Epub 2012 Apr 9. J Biol Chem. 2012. PMID: 22493484 Free PMC article.
References
-
- Ahmed Y, Hayashi S, Levine A, Wieschaus E (1998) Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93: 1171–1182 - PubMed
-
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307: 935–939 - PubMed
-
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM (2000) Drosophila p53 binds a damage response element at the reaper locus. Cell 101: 103–113 - PubMed
-
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

