Manipulation of an innate escape response in Drosophila: photoexcitation of acj6 neurons induces the escape response
- PMID: 19340304
- PMCID: PMC2660433
- DOI: 10.1371/journal.pone.0005100
Manipulation of an innate escape response in Drosophila: photoexcitation of acj6 neurons induces the escape response
Abstract
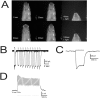
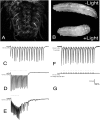
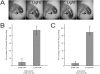
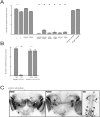
Background: The genetic analysis of behavior in Drosophila melanogaster has linked genes controlling neuronal connectivity and physiology to specific neuronal circuits underlying a variety of innate behaviors. We investigated the circuitry underlying the adult startle response, using photoexcitation of neurons that produce the abnormal chemosensory jump 6 (acj6) transcription factor. This transcription factor has previously been shown to play a role in neuronal pathfinding and neurotransmitter modality, but the role of acj6 neurons in the adult startle response was largely unknown.
Principal findings: We show that the activity of these neurons is necessary for a wild-type startle response and that excitation is sufficient to generate a synthetic escape response. Further, we show that this synthetic response is still sensitive to the dose of acj6 suggesting that that acj6 mutation alters neuronal activity as well as connectivity and neurotransmitter production.
Results/significance: These results extend the understanding of the role of acj6 and of the adult startle response in general. They also demonstrate the usefulness of activity-dependent characterization of neuronal circuits underlying innate behaviors in Drosophila, and the utility of integrating genetic analysis into modern circuit analysis techniques.
Conflict of interest statement
Figures
Similar articles
-
Abnormal chemosensory jump 6 is a positive transcriptional regulator of the cholinergic gene locus in Drosophila olfactory neurons.J Neurosci. 2002 Jul 1;22(13):5291-9. doi: 10.1523/JNEUROSCI.22-13-05291.2002. J Neurosci. 2002. PMID: 12097480 Free PMC article.
-
acj6: a gene affecting olfactory physiology and behavior in Drosophila.Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5467-71. doi: 10.1073/pnas.88.12.5467. Proc Natl Acad Sci U S A. 1991. PMID: 1905022 Free PMC article.
-
Neural circuits underlying context-dependent competition between defensive actions in Drosophila larvae.Nat Commun. 2025 Jan 28;16(1):1120. doi: 10.1038/s41467-025-56185-2. Nat Commun. 2025. PMID: 39875414 Free PMC article.
-
Systems neuroscience in Drosophila: Conceptual and technical advantages.Neuroscience. 2015 Jun 18;296:3-14. doi: 10.1016/j.neuroscience.2014.06.035. Epub 2014 Jun 25. Neuroscience. 2015. PMID: 24973655 Review.
-
Neural circuits that drive startle behavior, with a focus on the Mauthner cells and spiral fiber neurons of fishes.J Neurogenet. 2016 Jun;30(2):89-100. doi: 10.1080/01677063.2016.1182526. Epub 2016 Jun 14. J Neurogenet. 2016. PMID: 27302612 Review.
Cited by
-
The development and application of optogenetics.Annu Rev Neurosci. 2011;34:389-412. doi: 10.1146/annurev-neuro-061010-113817. Annu Rev Neurosci. 2011. PMID: 21692661 Free PMC article. Review.
-
Optogenetic manipulation of neural circuits and behavior in Drosophila larvae.Nat Protoc. 2012 Jul 12;7(8):1470-8. doi: 10.1038/nprot.2012.079. Nat Protoc. 2012. PMID: 22790083 Free PMC article.
-
Genetic manipulation of genes and cells in the nervous system of the fruit fly.Neuron. 2011 Oct 20;72(2):202-30. doi: 10.1016/j.neuron.2011.09.021. Neuron. 2011. PMID: 22017985 Free PMC article.
-
Genetics on the Fly: A Primer on the Drosophila Model System.Genetics. 2015 Nov;201(3):815-42. doi: 10.1534/genetics.115.183392. Genetics. 2015. PMID: 26564900 Free PMC article.
-
Studying Cerebellar Circuits by Remote Control of Selected Neuronal Types with GABA(A) Receptors.Front Mol Neurosci. 2009 Dec 11;2:29. doi: 10.3389/neuro.02.029.2009. eCollection 2009. Front Mol Neurosci. 2009. PMID: 20076763 Free PMC article.
References
-
- Naderi S, Ture U, Pait TG. History of the spinal cord localization. Neurosurg Focus. 2004;16:E15. - PubMed
-
- Brazier MA. The Abbe Nollet (1700–1770): the beginnings of electrotherapy. J Hist Neurosci. 1993;2:53–64. - PubMed
-
- Piccolino M. Luigi Galvani's path to animal electricity. C R Biol. 2006;329:303–318. - PubMed
-
- Shibata S, Oomura Y, Hattori K, Kita H. Responses of suprachiasmatic nucleus neurons to optic nerve stimulation in rat hypothalamic slice preparation. Brain Res. 1984;302:83–89. - PubMed
-
- Salzman CD, Britten KH, Newsome WT. Cortical microstimulation influences perceptual judgements of motion direction. Nature. 1990;346:174–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

