Somatic LKB1 mutations promote cervical cancer progression
- PMID: 19340305
- PMCID: PMC2660434
- DOI: 10.1371/journal.pone.0005137
Somatic LKB1 mutations promote cervical cancer progression
Abstract
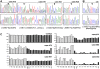
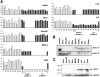
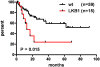
Human Papilloma Virus (HPV) is the etiologic agent for cervical cancer. Yet, infection with HPV is not sufficient to cause cervical cancer, because most infected women develop transient epithelial dysplasias that spontaneously regress. Progression to invasive cancer has been attributed to diverse host factors such as immune or hormonal status, as no recurrent genetic alterations have been identified in cervical cancers. Thus, the pressing question as to the biological basis of cervical cancer progression has remained unresolved, hampering the development of novel therapies and prognostic tests. Here we show that at least 20% of cervical cancers harbor somatically-acquired mutations in the LKB1 tumor suppressor. Approximately one-half of tumors with mutations harbored single nucleotide substitutions or microdeletions identifiable by exon sequencing, while the other half harbored larger monoallelic or biallelic deletions detectable by multiplex ligation probe amplification (MLPA). Biallelic mutations were identified in most cervical cancer cell lines; HeLa, the first human cell line, harbors a homozygous 25 kb deletion that occurred in vivo. LKB1 inactivation in primary tumors was associated with accelerated disease progression. Median survival was only 13 months for patients with LKB1-deficient tumors, but >100 months for patients with LKB1-wild type tumors (P = 0.015, log rank test; hazard ratio = 0.25, 95% CI = 0.083 to 0.77). LKB1 is thus a major cervical tumor suppressor, demonstrating that acquired genetic alterations drive progression of HPV-induced dysplasias to invasive, lethal cancers. Furthermore, LKB1 status can be exploited clinically to predict disease recurrence.
Conflict of interest statement
Figures


References
-
- Schoell WM, Janicek MF, Mirhashemi R. Epidemiology and biology of cervical cancer. Semin Surg Oncol. 1999;16:203–211. - PubMed
-
- Steben M, Duarte-Franco E. Human papillomavirus infection: epidemiology and pathophysiology. Gynecol Oncol. 2007;107:S2–5. - PubMed
-
- Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–538. - PubMed
-
- Crum CP, Lee KR. Diagnostic Gynecologic and Obstetric Pathology. Carlsbad, CA: Saunders; 2006.
-
- Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, et al. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111:167–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

