The opposite roles of nNOS in cardiac ischemia-reperfusion-induced injury and in ischemia preconditioning-induced cardioprotection in mice
- PMID: 19340535
- PMCID: PMC10717319
- DOI: 10.1007/s12576-009-0030-1
The opposite roles of nNOS in cardiac ischemia-reperfusion-induced injury and in ischemia preconditioning-induced cardioprotection in mice
Abstract
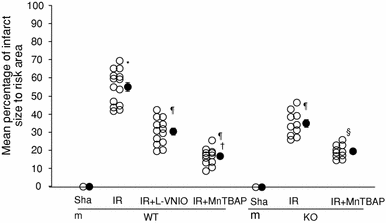
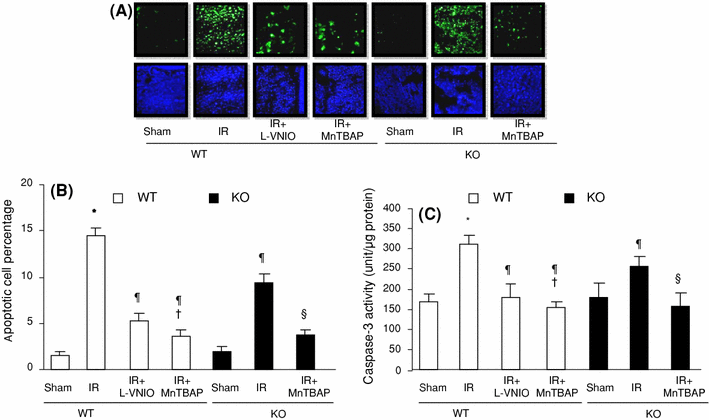
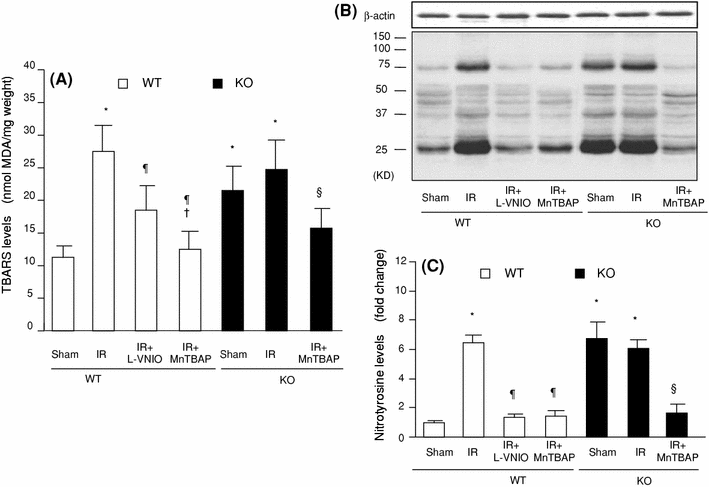
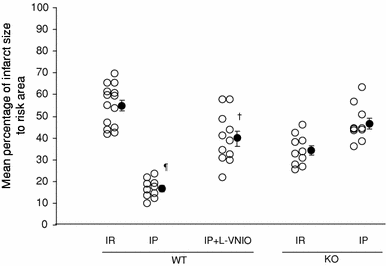
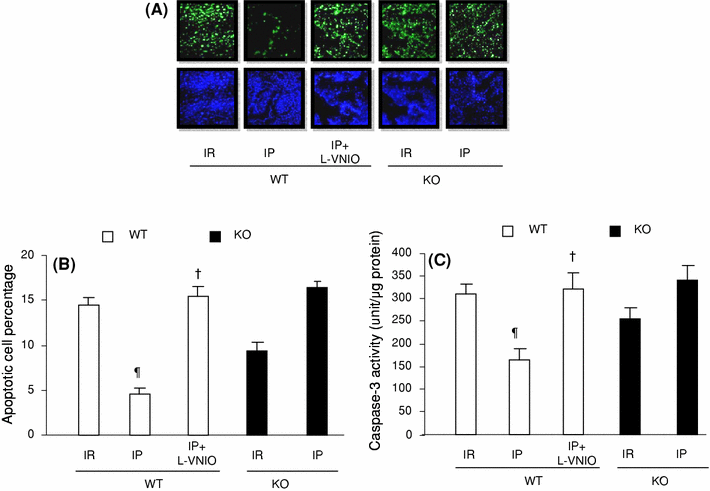
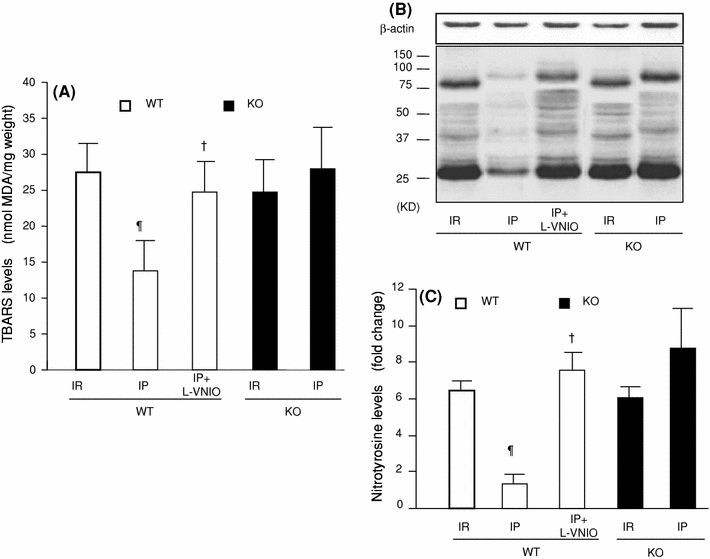
The role of neuronal nitric oxide synthase (nNOS) in cardiac ischemia-reperfusion (IR) and ischemia preconditioning (IP) is still controversial. Here, we focused on the possible roles of nNOS in cardiac IR and IP. Wild type C57BL/6 (WT) mice were subjected to coronary artery occlusion for 30 min followed by 24-h reperfusion (IR). Cardiac injury (infarct size and apoptotic cell number) was increased, associated with elevation of oxidative stress (lipid peroxidation) and nitrative stress (nitrotyrosine formation). A potent nNOS inhibitor, L-VNIO, and a superoxide dismutase mimetic and peroxynitrite scavenger, MnTBAP, significantly reduced IR-induced increases of oxidative/nitrative stress and cardiac injury. IR-induced cardiac injury in nNOS(-/-) (KO) mice was significantly lower than that in WT mice. MnTBAP markedly reduced IR-induced cardiac injury by suppression of oxidative/nitrative stress in KO mice. Cardiac IP was performed by three cycles of 5-min IR before 30-min ischemia followed by 24-h reperfusion. IP attenuated IR-induced cardiac injury in WT mice associated with reductions of oxidative/nitrative stress. IP-induced reduction of cardiac injury and oxidative/nitrative stress were eliminated by pretreatment with L-VNIO. In contrast with WT mice, IP had no protective effects in nNOS KO mice. In conclusion, nNOS played a dual role during cardiac IR and IP; nNOS exacerbated IR-induced injury by increasing oxidative/nitrative stress and contributed to IP-induced protection by inhibition of oxidative/nitrative stress.
Figures
Similar articles
-
Hypertrophied myocardium is vulnerable to ischemia/reperfusion injury and refractory to rapamycin-induced protection due to increased oxidative/nitrative stress.Clin Sci (Lond). 2018 Jan 11;132(1):93-110. doi: 10.1042/CS20171471. Print 2018 Jan 16. Clin Sci (Lond). 2018. PMID: 29175946
-
Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress.Circulation. 2007 Mar 20;115(11):1408-16. doi: 10.1161/CIRCULATIONAHA.106.666941. Epub 2007 Mar 5. Circulation. 2007. PMID: 17339545
-
Catalpol decreases peroxynitrite formation and consequently exerts cardioprotective effects against ischemia/reperfusion insult.Pharm Biol. 2013 Apr;51(4):463-73. doi: 10.3109/13880209.2012.740052. Epub 2013 Jan 22. Pharm Biol. 2013. PMID: 23336403
-
Role of nNOS in cardiac ischemia-reperfusion injury.Trends Cardiovasc Med. 2011 Feb;21(2):58-63. doi: 10.1016/j.tcm.2012.03.001. Trends Cardiovasc Med. 2011. PMID: 22578242 Review.
-
Pivotal role of nitric oxide in delayed pharmacological preconditioning against myocardial infarction.Toxicology. 2000 Nov 30;155(1-3):37-44. doi: 10.1016/s0300-483x(00)00275-4. Toxicology. 2000. PMID: 11154795 Review.
Cited by
-
Essential role of nitric oxide in acute ischemic preconditioning: S-nitros(yl)ation versus sGC/cGMP/PKG signaling?Free Radic Biol Med. 2013 Jan;54:105-12. doi: 10.1016/j.freeradbiomed.2012.09.005. Epub 2012 Sep 16. Free Radic Biol Med. 2013. PMID: 22989471 Free PMC article.
-
Nitrite as a mediator of ischemic preconditioning and cytoprotection.Nitric Oxide. 2011 Aug 1;25(2):70-80. doi: 10.1016/j.niox.2011.01.003. Epub 2011 Jan 26. Nitric Oxide. 2011. PMID: 21277988 Free PMC article. Review.
-
On the selectivity of neuronal NOS inhibitors.Br J Pharmacol. 2013 Mar;168(5):1255-65. doi: 10.1111/bph.12016. Br J Pharmacol. 2013. PMID: 23072468 Free PMC article.
-
Roles of endothelial nitric oxide synthase (eNOS) and mitochondrial permeability transition pore (MPTP) in epoxyeicosatrienoic acid (EET)-induced cardioprotection against infarction in intact rat hearts.J Mol Cell Cardiol. 2013 Jun;59:20-9. doi: 10.1016/j.yjmcc.2013.02.003. Epub 2013 Feb 16. J Mol Cell Cardiol. 2013. PMID: 23419451 Free PMC article.
-
Dual role of nNOS in ischemic injury and preconditioning.BMC Physiol. 2010 Aug 13;10:15. doi: 10.1186/1472-6793-10-15. BMC Physiol. 2010. PMID: 20707900 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

