Reduced volume-regulated outwardly rectifying anion channel activity in ventricular myocyte of type 1 diabetic mice
- PMID: 19340548
- PMCID: PMC10717248
- DOI: 10.1007/s12576-008-0012-8
Reduced volume-regulated outwardly rectifying anion channel activity in ventricular myocyte of type 1 diabetic mice
Abstract
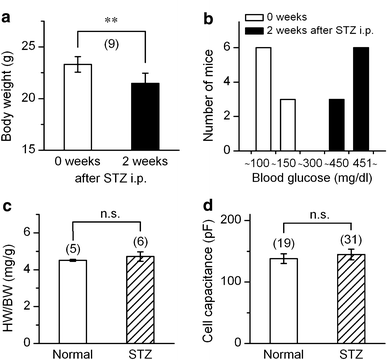
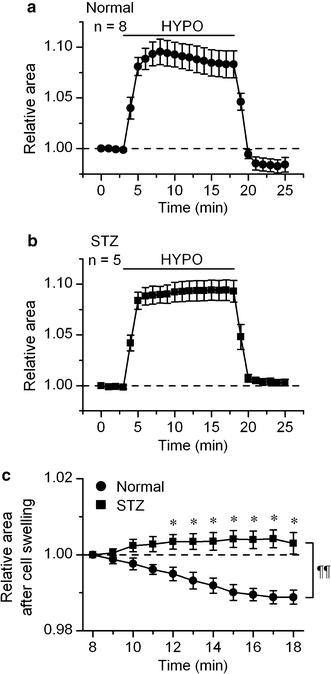
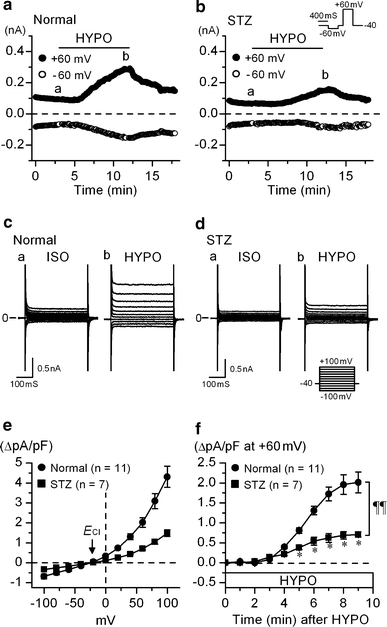
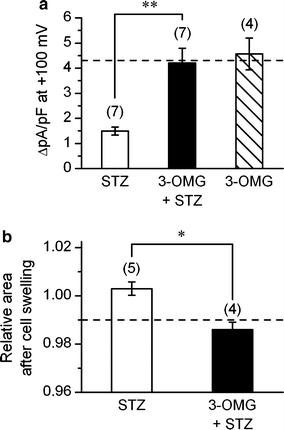
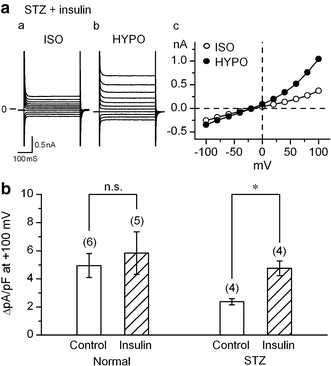
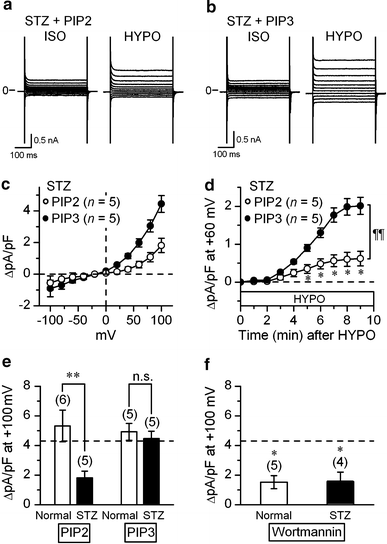
The currents through the volume-regulated outwardly rectifying anion channel (VRAC) were measured in single ventricular myocytes obtained from streptozotocin (STZ)-induced diabetic mice, using whole-cell voltage-clamp method. In myocytes from STZ-diabetic mice, the density of VRAC current induced by hypotonic perfusion was markedly reduced, compared with that in the cells form normal control mice. Video-image analysis showed that the regulatory volume decrease (RVD), which was seen in normal cells after osmotic swelling, was almost lost in myocytes from STZ-diabetic mice. Some mice were pretreated with 3-O-methylglucose before STZ injection, to prevent the STZ's beta cell toxicity. In the myocytes obtained from such mice, the magnitude of VRAC current and the degree of RVD seen during hypotonic challenge were almost normal. Incubation of the myocytes from STZ-diabetic mice with insulin reversed the attenuation of VRAC current. These findings suggested that the STZ-induced chronic insulin-deficiency was an important causal factor for the attenuation of VRAC current. Intracellular loading of the STZ-diabetic myocytes with phosphatidylinositol 3,4,5-trisphosphate (PIP3), but not phosphatidylinositol 4,5-bisphosphate (PIP2), also reversed the attenuation of VRAC current. Furthermore, treatment of the normal cells with wortmannin, a phosphatidylinositol 3-kinase (PI3K) inhibitor, suppressed the development of VRAC current. We postulate that an impairment PI3K-PIP3 pathway, which may be insulin-dependent, is responsible for the attenuation of VRAC currents in STZ-diabetic myocytes.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

