The maxi-anion channel: a classical channel playing novel roles through an unidentified molecular entity
- PMID: 19340557
- PMCID: PMC10717152
- DOI: 10.1007/s12576-008-0008-4
The maxi-anion channel: a classical channel playing novel roles through an unidentified molecular entity
Abstract
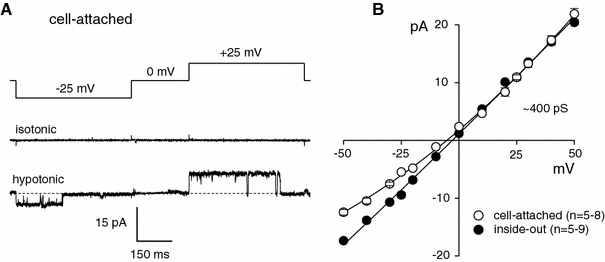
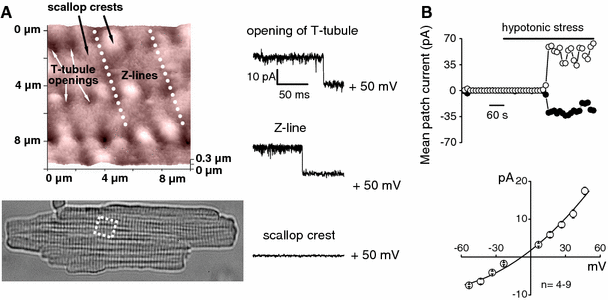
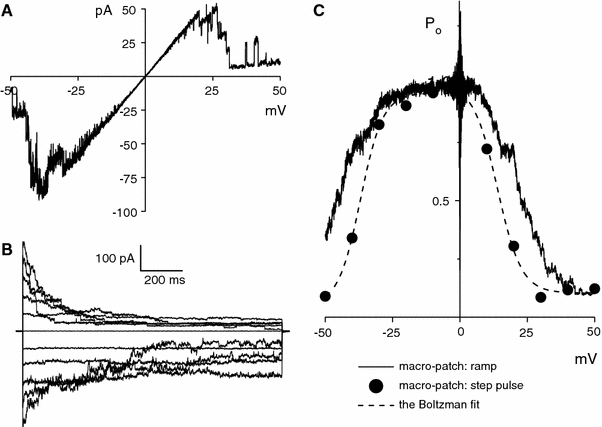
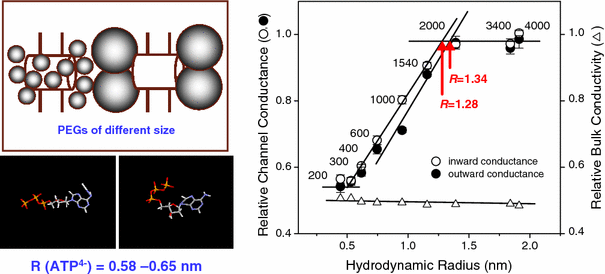
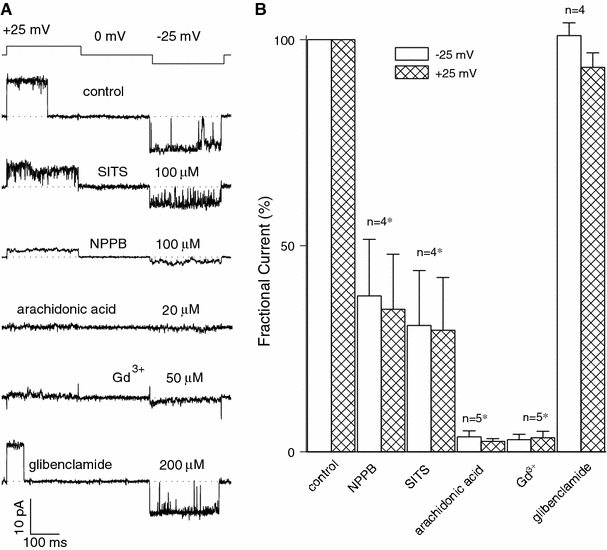
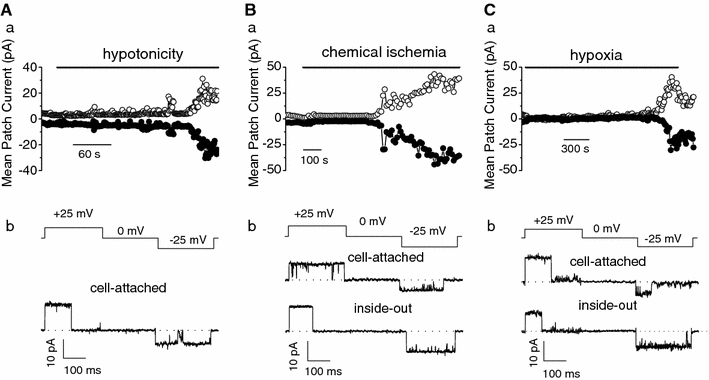
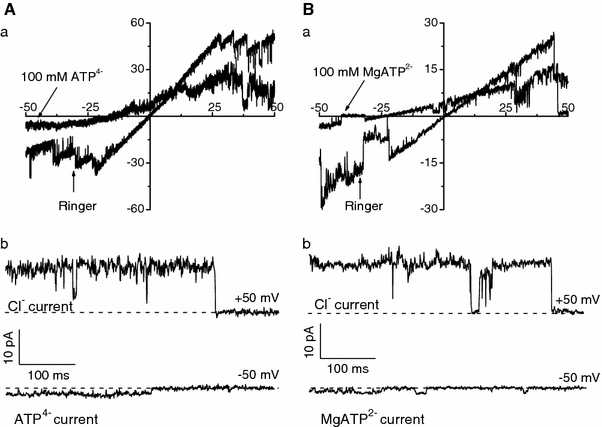
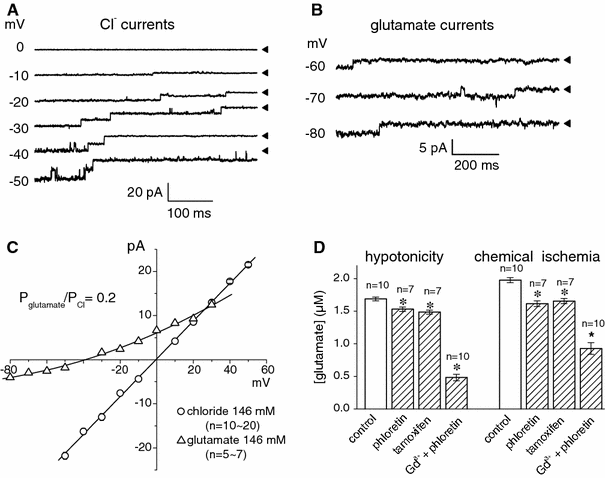
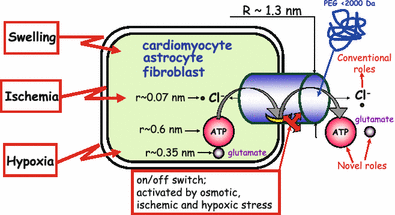
The maxi-anion channel is widely expressed and found in almost every part of the body. The channel is activated in response to osmotic cell swelling, to excision of the membrane patch, and also to some other physiologically and pathophysiologically relevant stimuli, such as salt stress in kidney macula densa as well as ischemia/hypoxia in heart and brain. Biophysically, the maxi-anion channel is characterized by a large single-channel conductance of 300-400 pS, which saturates at 580-640 pS with increasing the Cl(-) concentration. The channel discriminates well between Na(+) and Cl(-), but is poorly selective to other halides exhibiting weak electric-field selectivity with an Eisenman's selectivity sequence I. The maxi-anion channel has a wide pore with an effective radius of approximately 1.3 nm and permits passage not only of Cl(-) but also of some intracellular large organic anions, thereby releasing major extracellular signals and gliotransmitters such as glutamate(-) and ATP(4-). The channel-mediated efflux of these signaling molecules is associated with kidney tubuloglomerular feedback, cardiac ischemia/hypoxia, as well as brain ischemia/hypoxia and excitotoxic neurodegeneration. Despite the ubiquitous expression, well-defined properties and physiological/pathophysiological significance of this classical channel, the molecular entity has not been identified. Molecular identification of the maxi-anion channel is an urgent task that would greatly promote investigation in the fields not only of anion channel but also of physiological/pathophysiological signaling in the brain, heart and kidney.
Figures
Similar articles
-
Molecular Identities and ATP Release Activities of Two Types of Volume-Regulatory Anion Channels, VSOR and Maxi-Cl.Curr Top Membr. 2018;81:125-176. doi: 10.1016/bs.ctm.2018.07.004. Epub 2018 Aug 17. Curr Top Membr. 2018. PMID: 30243431 Review.
-
ATP-conducting maxi-anion channel: a new player in stress-sensory transduction.Jpn J Physiol. 2004 Feb;54(1):7-14. doi: 10.2170/jjphysiol.54.7. Jpn J Physiol. 2004. PMID: 15040843 Review.
-
The properties, functions, and pathophysiology of maxi-anion channels.Pflugers Arch. 2016 Mar;468(3):405-20. doi: 10.1007/s00424-015-1774-5. Epub 2016 Jan 6. Pflugers Arch. 2016. PMID: 26733413 Review.
-
Maxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cells.Am J Physiol Cell Physiol. 2012 Nov 1;303(9):C924-35. doi: 10.1152/ajpcell.00459.2011. Epub 2012 Jul 11. Am J Physiol Cell Physiol. 2012. PMID: 22785119
-
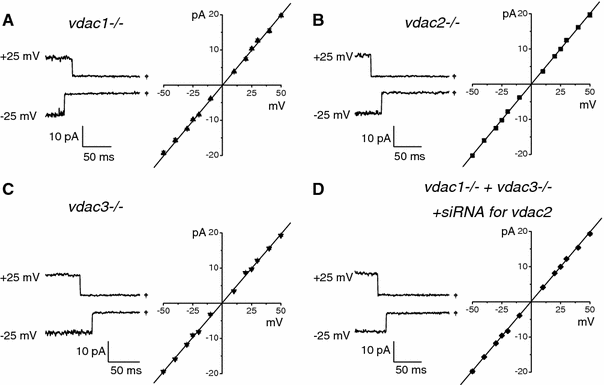
Plasmalemmal VDAC controversies and maxi-anion channel puzzle.Biochim Biophys Acta. 2012 Jun;1818(6):1570-80. doi: 10.1016/j.bbamem.2011.09.024. Epub 2011 Oct 1. Biochim Biophys Acta. 2012. PMID: 21986486 Review.
Cited by
-
Swelling-activated anion channels are essential for volume regulation of mouse thymocytes.Int J Mol Sci. 2011;12(12):9125-37. doi: 10.3390/ijms12129125. Epub 2011 Dec 8. Int J Mol Sci. 2011. PMID: 22272123 Free PMC article.
-
Gating of maxi channels observed from pseudo-phase portraits.Am J Physiol Cell Physiol. 2013 Mar 1;304(5):C450-7. doi: 10.1152/ajpcell.00378.2012. Epub 2013 Jan 2. Am J Physiol Cell Physiol. 2013. PMID: 23283936 Free PMC article.
-
Imaging single photons and intrinsic optical signals for studies of vesicular and non-vesicular ATP release from axons.Front Neuroanat. 2011 Jun 6;5:32. doi: 10.3389/fnana.2011.00032. eCollection 2011. Front Neuroanat. 2011. PMID: 21852965 Free PMC article.
-
Measuring membrane protein dynamics in neurons using fluorescence recovery after photobleach.Methods Enzymol. 2012;504:127-46. doi: 10.1016/B978-0-12-391857-4.00006-9. Methods Enzymol. 2012. PMID: 22264532 Free PMC article. Review.
-
Epithelia of the ovine and bovine forestomach express basolateral maxi-anion channels permeable to the anions of short-chain fatty acids.Pflugers Arch. 2014 Sep;466(9):1689-712. doi: 10.1007/s00424-013-1386-x. Epub 2013 Nov 17. Pflugers Arch. 2014. PMID: 24240698
References
-
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol. 1997;273:C755–C789. - PubMed
-
- Jalonen T. Single-channel characteristics of the large-conductance anion channel in rat cortical astrocytes in primary culture. Glia. 1993;9:227–237. - PubMed
-
- Liu HT, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia. 2006;54:343–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

