Acrolein-derived DNA adduct formation in human colon cancer cells: its role in apoptosis induction by docosahexaenoic acid
- PMID: 19341237
- PMCID: PMC2683896
- DOI: 10.1021/tx800355k
Acrolein-derived DNA adduct formation in human colon cancer cells: its role in apoptosis induction by docosahexaenoic acid
Abstract
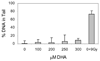
The apoptotic effects of docosahexaenoic acid (DHA) and other omega-3 polyunsaturated fatty acids (PUFAs) have been documented in cell and animal studies. The molecular mechanism by which DHA induces apoptosis is unclear. Although there is no direct evidence, some studies have suggested that DNA damage generated through lipid peroxidation may be involved. Our previous studies showed that DHA, because it has a high degree of unsaturation, can give rise to the acrolein-derived 1,N(2)-propanodeoxyguanosine (Acr-dG) as a major class of DNA adducts via lipid oxidation. As a first step to investigate the possible role of oxidative DNA damage in apoptosis induced by DHA, we examined the relationships between oxidative DNA damage and apoptosis caused by DHA in human colon cancer HT-29 cells. Apoptosis and oxidative DNA damage, including Acr-dG and 8-oxo-deoxyguanosine (8-oxo-dG) formation, in cells treated with DHA and omega-6 PUFAs, including arachidonic acid (AA) and linoleic acid (LA), were measured. DHA induced apoptosis in a dose- and time-dependent manner with a concentration range from 0 to 300 microM as indicated by increased caspase-3 activity and PARP cleavage. In contrast, AA and LA had little or no effect at these concentrations. The Acr-dG levels were increased in HT-29 cells treated with DHA at 240 and 300 microM, and the increases were correlated with the induction of apoptosis at these concentrations, while no significant changes were observed for 8-oxo-dG. Because proteins may compete with DNA to react with acrolein, we then examined the effects of BSA on DHA-induced apoptosis and oxidative DNA damage. The addition of BSA to HT-29 cell culture media significantly decreases Acr-dG levels with a concomitant decrease in the apoptosis induced by DHA. The reduced Acr-dG formation is attributed to the reaction of BSA with acrolein as indicated by increased levels of total protein carbonyls. Similar correlations between Acr-dG formation and apoptosis were observed in HT-29 cells directly incubated with 0-200 microM acrolein. Additionally, DHA treatment increased the level of DNA strand breaks and caused cell cycle arrested at G1 phase. Taken together, these results demonstrate the parallel relationships between Acr-dG level and apoptosis in HT-29 cells, suggesting that the formation of Acr-dG in cellular DNA may contribute to apoptosis induced by DHA.
Figures






References
-
- Deschner EE, Lytle JS, Wong G, Ruperto JF, Newmark HL. The effect of dietary omega-3-fatty-acids (fish oil) on azoxymethanol-induced focal areas of dysplasia and colon-tumor incidence. Cancer. 1990;66(11):2350–2356. - PubMed
-
- Chang WCL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. Journal of Nutrition. 1998;128(3):491–497. - PubMed
-
- Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. American Journal of Clinical Nutrition. 1999;70(1):85–90. - PubMed
-
- Rao CV, Hirose Y, Indranie C, Reddy BS. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Research. 2001;61(5):1927–1933. - PubMed
-
- Kimura Y, Kono S, Toyomura K, Nagano J, Mizoue T, Moore MA, Mibu R, Tanaka M, Kakeji Y, Maehara Y, Okamura T, Ikejiri K, Futami K, Yasunami Y, Maekawa T, Takenaka K, Ichimiya H, Imaizumi N. Meat, fish and fat intake in relation to subsite-specific risk of colorectal cancer: The Fukuoka Colorectal Cancer Study. Cancer Science. 2007;98(4):590–597. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

