Monitoring phosphorylation of the pyruvate dehydrogenase complex
- PMID: 19341700
- PMCID: PMC2713743
- DOI: 10.1016/j.ab.2009.03.040
Monitoring phosphorylation of the pyruvate dehydrogenase complex
Abstract
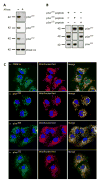
The pyruvate dehydrogenase multienzyme complex (PDC) is a key regulatory point in cellular metabolism linking glycolysis to the citric acid cycle and lipogenesis. Reversible phosphorylation of the pyruvate dehydrogenase enzyme is a critical regulatory mechanism and an important point for monitoring metabolic activity. To directly determine the regulation of the PDC by phosphorylation, we developed a complete set of phospho-antibodies against the three known phosphorylation sites on the E1 alpha subunit of pyruvate dehydrogenase (PDHE1alpha). We demonstrate phospho-site specificity of each antibody in a variety of cultured cells and tissue extracts. In addition, we show sensitivity of these antibodies to PDH activity using the pyruvate dehydrogenase kinase-specific inhibitor dichloroacetate. We go on to use these antibodies to assess PDH phosphorylation in a patient suffering from Leigh's syndrome. Finally, we observe changes in individual phosphorylation states following a small molecule screen, demonstrating that these reagents should be useful for monitoring phosphorylation of PDHE1alpha and, therefore, overall metabolism in the disease state as well as in response to a myriad of physiological and pharmacological stimuli.
Figures




Similar articles
-
Studies on the regulation of the mitochondrial alpha-ketoacid dehydrogenase complexes and their kinases.Adv Enzyme Regul. 1997;37:271-93. doi: 10.1016/s0065-2571(96)00009-x. Adv Enzyme Regul. 1997. PMID: 9381974
-
Characterization of two cDNA clones for pyruvate dehydrogenase E1 beta subunit and its regulation in tricarboxylic acid cycle-deficient fibroblast.J Biol Chem. 1990 Aug 5;265(22):13320-6. J Biol Chem. 1990. PMID: 2376596
-
Deficiency of pyruvate dehydrogenase complex (PDHC) in Leigh's disease fibroblasts: an abnormality in lipoamide dehydrogenase affecting PDHC activation.Neurology. 1989 Jan;39(1):70-5. doi: 10.1212/wnl.39.1.70. Neurology. 1989. PMID: 2909916
-
Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer.J Natl Cancer Inst. 2017 Nov 1;109(11). doi: 10.1093/jnci/djx071. J Natl Cancer Inst. 2017. PMID: 29059435 Review.
-
Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: complexity of multiple phosphorylation sites and kinases.Exp Mol Med. 2001 Dec 31;33(4):191-7. doi: 10.1038/emm.2001.32. Exp Mol Med. 2001. PMID: 11795479 Review.
Cited by
-
Metabolic reprogramming: A novel therapeutic target in diabetic kidney disease.Front Pharmacol. 2022 Sep 2;13:970601. doi: 10.3389/fphar.2022.970601. eCollection 2022. Front Pharmacol. 2022. PMID: 36120335 Free PMC article. Review.
-
Consequences of reprogramming acetyl-CoA metabolism by 2,3,7,8-tetrachlorodibenzo-p-dioxin in the mouse liver.Sci Rep. 2023 Mar 13;13(1):4138. doi: 10.1038/s41598-023-31087-9. Sci Rep. 2023. PMID: 36914879 Free PMC article.
-
Effects of octreotide on autophagy markers and cell viability markers related to metabolic activity in rat pituitary tumor cells.Pituitary. 2020 Jun;23(3):223-231. doi: 10.1007/s11102-020-01028-0. Pituitary. 2020. PMID: 31997055
-
A comparison of phosphospecific affinity reagents reveals the utility of recombinant Forkhead-associated domains in recognizing phosphothreonine-containing peptides.N Biotechnol. 2016 Sep 25;33(5 Pt A):537-43. doi: 10.1016/j.nbt.2015.12.006. Epub 2016 Jan 6. N Biotechnol. 2016. PMID: 26772725 Free PMC article.
-
Therapeutic arginine starvation in ASS1-deficient cancers inhibits the Warburg effect.Mol Cell Oncol. 2017 Mar 1;4(3):e1295131. doi: 10.1080/23723556.2017.1295131. eCollection 2017. Mol Cell Oncol. 2017. PMID: 28616574 Free PMC article.
References
-
- Harris RA, Bowker-Kinley MM, Huang B, Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul. 2002;42:249–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

