A missense mutation in the Capza3 gene and disruption of F-actin organization in spermatids of repro32 infertile male mice
- PMID: 19341723
- PMCID: PMC2688473
- DOI: 10.1016/j.ydbio.2009.03.020
A missense mutation in the Capza3 gene and disruption of F-actin organization in spermatids of repro32 infertile male mice
Abstract
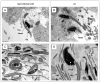
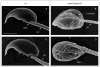
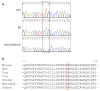
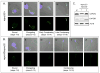
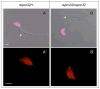
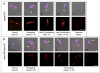
Males homozygous for the repro32 ENU-induced mutation produced by the Reproductive Genomics program at The Jackson Laboratory are infertile, have low epididymal sperm concentrations, and produce sperm with abnormally shaped heads and poor motility. The purpose of the present study was to identify the mutated gene in repro32 mice and to define the structural and functional changes causing infertility and the aberrant sperm phenotype. In repro32/repro32 mice, we discovered a failure to shed excess cytoplasm and disorganization of the middle piece of the flagellum at spermiation, resulting in the outer dense fibers being wrapped around the sperm head within a bag of cytoplasm. Using a candidate-gene approach, a mutation was identified in the spermatid-specific "capping protein (actin filament) muscle Z-line, alpha 3" gene (Capza3). CAPZA3 protein localization was altered in spermatids concurrent with altered localization of a unique CAPZB variant isoform and disruption of the filamentous actin (F-actin) network. These observations strongly suggest the missense mutation in Capza3 is responsible for the mutant phenotype of repro32/repro32 sperm and regulation of F-actin dynamics by a spermatogenic cell-specific CAPZ heterodimer is essential for removal of the cytoplasm and maintenance of midpiece integrity during spermiation in the mouse.
Figures


Similar articles
-
Phospholipase C zeta (PLCζ) and male infertility: Clinical update and topical developments.Adv Biol Regul. 2016 May;61:58-67. doi: 10.1016/j.jbior.2015.11.009. Epub 2015 Nov 30. Adv Biol Regul. 2016. PMID: 26700242 Review.
-
Analysis of CAPZA3 localization reveals temporally discrete events during the acrosome reaction.J Cell Physiol. 2010 Sep;224(3):575-80. doi: 10.1002/jcp.22211. J Cell Physiol. 2010. PMID: 20458735 Free PMC article.
-
Loss of TSLC1 causes male infertility due to a defect at the spermatid stage of spermatogenesis.Mol Cell Biol. 2006 May;26(9):3595-609. doi: 10.1128/MCB.26.9.3595-3609.2006. Mol Cell Biol. 2006. PMID: 16611999 Free PMC article.
-
Sperm Release at Spermiation Is Regulated by Changes in the Organization of Actin- and Microtubule-Based Cytoskeletons at the Apical Ectoplasmic Specialization-A Study Using the Adjudin Model.Endocrinology. 2017 Dec 1;158(12):4300-4316. doi: 10.1210/en.2017-00660. Endocrinology. 2017. PMID: 29040437 Free PMC article.
-
Haploid male germ cells-the Grand Central Station of protein transport.Hum Reprod Update. 2020 Jun 18;26(4):474-500. doi: 10.1093/humupd/dmaa004. Hum Reprod Update. 2020. PMID: 32318721 Review.
Cited by
-
Mouse models in male fertility research.Asian J Androl. 2011 Jan;13(1):139-51. doi: 10.1038/aja.2010.101. Epub 2010 Nov 8. Asian J Androl. 2011. PMID: 21057516 Free PMC article. Review.
-
SETDB1 Links the Meiotic DNA Damage Response to Sex Chromosome Silencing in Mice.Dev Cell. 2018 Dec 3;47(5):645-659.e6. doi: 10.1016/j.devcel.2018.10.004. Epub 2018 Nov 1. Dev Cell. 2018. PMID: 30393076 Free PMC article.
-
Loss of CEP70 function affects acrosome biogenesis and flagella formation during spermiogenesis.Cell Death Dis. 2021 May 12;12(5):478. doi: 10.1038/s41419-021-03755-z. Cell Death Dis. 2021. PMID: 33980814 Free PMC article.
-
Diagnosis of genetic defects through parallel assessment of PLCζ and CAPZA3 in infertile men with history of failed oocyte activation.Iran J Basic Med Sci. 2016 Mar;19(3):281-9. Iran J Basic Med Sci. 2016. PMID: 27114798 Free PMC article.
-
SPATC1L maintains the integrity of the sperm head-tail junction.EMBO Rep. 2018 Sep;19(9):e45991. doi: 10.15252/embr.201845991. Epub 2018 Jul 19. EMBO Rep. 2018. PMID: 30026308 Free PMC article.
References
-
- Beier DR, Herron BJ. Genetic mapping and ENU mutagenesis. Genetica. 2004;122:65–69. - PubMed
-
- Casella JF, Torres MA. Interaction of Cap Z with actin. The NH2-terminal domains of the alpha 1 and beta subunits are not required for actin capping, and alpha 1 beta and alpha 2 beta heterodimers bind differentially to actin. J Biol Chem. 1994;269(9):6992–6998. - PubMed
-
- Clermont Y, Oko R, Hermo L. Cell biology of mammalian spermiogenesis. In: Desjardins C, Ewing LL, editors. Cell and Molecular Biology of the Testis. Oxford University Press; New York: 2003. pp. 332–376.
-
- Cooper TG. Cytoplasmic droplets: the good, the bad, or just confusing? Hum Reprod. 2005;20:9–11. - PubMed
-
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

