Lentiviral delivery of short hairpin RNAs
- PMID: 19341774
- PMCID: PMC2789654
- DOI: 10.1016/j.addr.2009.03.004
Lentiviral delivery of short hairpin RNAs
Abstract
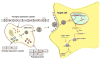
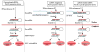
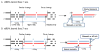
In less than a decade after discovery, RNA interference-mediated gene silencing is already being tested as potential therapy in clinical trials for a number of diseases. Lentiviral vectors provide a means to express short hairpin RNA (shRNA) to induce stable and long-term gene silencing in both dividing and non-dividing cells and thus, are being intensively investigated for this purpose. However, induction of long-term shRNA expression can also cause toxicities by inducing off-target effects and interference with the endogenous micro-RNA (miRNA) pathway that regulates cellular gene expression. Recently, several advances have been made in the shRNA vector design to mimic cellular miRNA processing and to express multiplex siRNAs in a tightly regulated and reversible manner to overcome toxicities. In this review we describe some of these advances, focusing on the progress made in the development of lentiviral shRNA delivery strategies to combat viral infections.
Figures
Similar articles
-
Expression of small hairpin RNA by lentivirus-based vector confers efficient and stable gene-suppression of HIV-1 on human cells including primary non-dividing cells.Microbes Infect. 2004 Jan;6(1):76-85. doi: 10.1016/j.micinf.2003.10.009. Microbes Infect. 2004. PMID: 14738896
-
Short-term cytotoxic effects and long-term instability of RNAi delivered using lentiviral vectors.BMC Mol Biol. 2004 Aug 3;5:9. doi: 10.1186/1471-2199-5-9. BMC Mol Biol. 2004. PMID: 15291968 Free PMC article.
-
Transduction with Lentiviral Vectors Altered the Expression Profile of Host MicroRNAs.J Virol. 2018 Aug 29;92(18):e00503-18. doi: 10.1128/JVI.00503-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29997205 Free PMC article.
-
Lentiviral vector-mediated gene transfer and RNA silencing technology in neuronal dysfunctions.Mol Biotechnol. 2011 Feb;47(2):169-87. doi: 10.1007/s12033-010-9334-x. Mol Biotechnol. 2011. PMID: 20862616 Review.
-
Lentiviral-mediated delivery of siRNAs for antiviral therapy.Gene Ther. 2006 Mar;13(6):553-8. doi: 10.1038/sj.gt.3302688. Gene Ther. 2006. PMID: 16397511 Free PMC article. Review.
Cited by
-
Viral Delivery of dsRNA for Control of Insect Agricultural Pests and Vectors of Human Disease: Prospects and Challenges.Front Physiol. 2017 Jun 14;8:399. doi: 10.3389/fphys.2017.00399. eCollection 2017. Front Physiol. 2017. PMID: 28659820 Free PMC article. Review.
-
An antibody fragment functionalized dendritic PEGylated poly(2-(dimethylamino)ethyl diacrylate) as a vehicle of exogenous microRNA.Drug Deliv Transl Res. 2012 Oct;2(5):406-14. doi: 10.1007/s13346-012-0097-8. Drug Deliv Transl Res. 2012. PMID: 25787178
-
Transfer and Expression of Small Interfering RNAs in Mammalian Cells Using Lentiviral Vectors.Acta Naturae. 2013 Apr;5(2):7-18. Acta Naturae. 2013. PMID: 23819033 Free PMC article.
-
Apolipoprotein B knockdown by AAV-delivered shRNA lowers plasma cholesterol in mice.Mol Ther. 2011 Apr;19(4):731-40. doi: 10.1038/mt.2011.6. Epub 2011 Feb 8. Mol Ther. 2011. PMID: 21304496 Free PMC article.
-
Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles.Horm Behav. 2013 Mar;63(3):518-26. doi: 10.1016/j.yhbeh.2013.01.005. Epub 2013 Jan 28. Horm Behav. 2013. PMID: 23370363 Free PMC article.
References
-
- Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. - PubMed
-
- Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–378. - PubMed
-
- Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. - PubMed
-
- Geiss G, Jin G, Guo J, Bumgarner R, Katze MG, Sen GC. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J Biol Chem. 2001;276:30178–30182. - PubMed
-
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

