Effects of aging and calorie restriction on rat skeletal muscle glycogen synthase and glycogen phosphorylase
- PMID: 19341787
- PMCID: PMC5127239
- DOI: 10.1016/j.exger.2009.03.005
Effects of aging and calorie restriction on rat skeletal muscle glycogen synthase and glycogen phosphorylase
Abstract
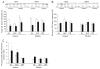
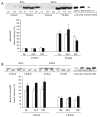
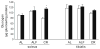
Calorie restriction's (CR) effects on age-associated changes in glycogen-metabolizing enzymes were studied in rat soleus (SOL) and tibialis anterior (TA) muscles. Old (24 months) compared to young (6 months) rats maintained ad libitum on a standard diet had reduced glycogen synthase (GS) activity, lower muscle GS protein levels, increased phosphorylation of GS at site 3a with less activation in SOL. Age-associated impairments in GS protein and activation-phosphorylation were also shown in TA. There was an age-associated reduction in glycogen phosphorylase (GP) activity level in SOL, while brain/muscle isoforms (B/M) of GP protein levels were higher. GP activity and protein levels were preserved, but GP was inactivated in TA with age. Glycogen content was unchanged in both muscles. CR did not alter GS or GP activity/protein levels in young rats. CR hindered age-related decreases in GS activity/protein, unrelated to GS mRNA levels, and GS inactivation-phosphorylation; not on GP. In older rats, CR enhanced glycogen accumulation in SOL. Short-term fasting did not recapitulate CR effects in old rats. Thus, the predominant age-associated impairments on skeletal muscle GS and GP activities occur in the oxidative SOL muscle of rats, and CR can attenuate the loss of GS activity/activation and stimulate glycogen accumulation.
Figures
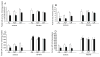
References
-
- Baqué S, Guinovart JJ, Gómez-Foix AM. Overexpression of muscle glycogen phosphorylase in cultured human muscle fibers causes increased glucose consumption and nonoxidative disposal. J Biol Chem. 1996;271:2594–2598. - PubMed
-
- Buchbinder JL, Rath VL, Fletterick RJ. Structural relationships among regulated and unregulated phosphorylases. Annu Rev Biophys Biomol Struct. 2001;30:191–209. - PubMed
-
- Crerar MM, Karlsson O, Fletterick RJ, Hwang PK. Chimeric muscle and brain glycogen phosphorylases define protein domains governing isozyme- specific responses to allosteric activation. J Biol Chem. 1995;270:13748–13756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

