Overexpression of catalase delays G0/G1- to S-phase transition during cell cycle progression in mouse aortic endothelial cells
- PMID: 19341793
- PMCID: PMC2713001
- DOI: 10.1016/j.freeradbiomed.2009.03.018
Overexpression of catalase delays G0/G1- to S-phase transition during cell cycle progression in mouse aortic endothelial cells
Abstract
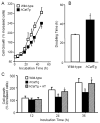
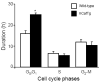
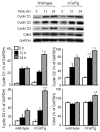
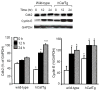
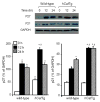
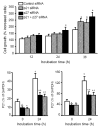
Although it is understood that hydrogen peroxide (H(2)O(2)) promotes cellular proliferation, little is known about its role in endothelial cell cycle progression. To assess the regulatory role of endogenously produced H(2)O(2) in cell cycle progression, we studied the cell cycle progression in mouse aortic endothelial cells (MAECs) obtained from mice overexpressing a human catalase transgene (hCatTg), which destroys H(2)O(2). The hCatTg MAECs displayed a prolonged doubling time compared to wild-type controls (44.0 +/- 4.7 h versus 28.6 +/- 0.8 h, p<0.05), consistent with a diminished growth rate and H(2)O(2) release. Incubation with aminotriazole, a catalase inhibitor, prevented the observed diminished growth rate in hCatTg MAECs. Inhibition of catalase activity with aminotriazole abrogated catalase overexpression-induced antiproliferative action. Flow cytometry analysis indicated that the prolonged doubling time was principally due to an extended G(0)/G(1) phase in hCatTg MAECs compared to the wild-type cells (25.0 +/- 0.9 h versus 15.9 +/- 1.4 h, p< 0.05). The hCatTg MAECs also exhibited decreased activities of the cyclin-dependent kinase (Cdk) complexes responsible for G(0)/G(1)- to S-phase transition in the cell cycle, including the cyclin D-Cdk4 and cyclin E-Cdk2 complexes. Moreover, the reduction in cyclin-Cdk activities in hCatTg MAECs was accompanied by increased protein levels of two Cdk inhibitors, p21 and p27, which inhibit the Cdk activity required for the G(0)/G(1)- to S-phase transition. Knockdown of p21 and/or p27 attenuated the antiproliferative effect of catalase overexpression in MAECs. These results, together with the fact that catalase is an H(2)O(2) scavenger, suggest that endogenously produced H(2)O(2) mediates MAEC proliferation by fostering the transition from G(0)/G(1) to S phase.
Conflict of interest statement
Figures



References
-
- Sueishi K, Yonemitsu Y, Nakagawa K, Kaneda Y, Kumamoto M, Nakashima Y. Atherosclerosis and angiogenesis. Its pathophysiological significance in humans as well as in an animal model induced by the gene transfer of vascular endothelial growth factor. Ann N Y Acad Sci. 1997;811:311–322. - PubMed
-
- Spyridopoulos I, Andres V. Control of vascular smooth muscle and endothelial cell proliferaiton and its implicaiton in cardiovascular disease. Front Biosci. 1998;3:269–287. - PubMed
-
- Yasuda M, Ohzeki Y, Shimizu S, Naito S, Ohtsuru A, Yamamoto T, Kuroiwa Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with Ets-1 in endothelial cells. Life Sci. 1998;64:249–258. - PubMed
-
- Ruiz-Gines JA, Lopez-Ongil S, Gonzalez-Rubio M, Gonzalez-Santiago L, Rodriguez-Puyol M, Rodriguez-Puyol D. Reactive oxygen species induce proliferation of bovine aortic endothelial cells. J Cardiovasc Pharmacol. 2000;35:109–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01HL-076623/HL/NHLBI NIH HHS/United States
- T32HL07735/HL/NHLBI NIH HHS/United States
- U54 CA091408/CA/NCI NIH HHS/United States
- F31 HL083921/HL/NHLBI NIH HHS/United States
- G12RR03032/RR/NCRR NIH HHS/United States
- F31HL083921/HL/NHLBI NIH HHS/United States
- T32 HL007735/HL/NHLBI NIH HHS/United States
- 5U54CA091408-07/CA/NCI NIH HHS/United States
- G12 RR003032/RR/NCRR NIH HHS/United States
- R01 ES014472/ES/NIEHS NIH HHS/United States
- R01ES014471/ES/NIEHS NIH HHS/United States
- K01 HL076623/HL/NHLBI NIH HHS/United States
- G12RR003032/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

