Gab2-mediated signaling promotes melanoma metastasis
- PMID: 19342374
- PMCID: PMC2671382
- DOI: 10.2353/ajpath.2009.080543
Gab2-mediated signaling promotes melanoma metastasis
Abstract
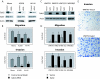
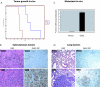
Metastatic melanoma is a disease with a poor prognosis that currently lacks effective treatments. Critical biological features of metastasis include acquisition of migratory competence, growth factor independence, and invasive potential. In an attempt to identify genes that contribute to melanoma pathogenesis, a genome-wide search using bacterial artificial chromosome array comparative genomic hybridization and single nucleotide polymorphism arrays in a series of 64 metastatic melanoma samples and 20 melanoma cell lines identified increased copy numbers of Gab2 located on 11q14.1. Gab2 is an adaptor protein that potentiates the activation of the Ras-Erk and PI3K-Akt pathways and has recently been implicated in human cancer; however, its role in melanoma has not been explored. In this study, we found that Gab2 was either amplified (approximately 11%) and/or overexpressed (approximately 50%) in melanoma. Gab2 protein expression correlated with clinical melanoma progression, and higher levels of expression were seen in metastatic melanomas compared with primary melanoma and melanocytic nevi. We found that overexpression of Gab2 potentiates, whereas silencing of Gab2 reduces, migration and invasion of melanoma cells. Gab2 mediated the hyperactivation of Akt signaling in the absence of growth factors, whereas inhibition of the PI3K-Akt pathway decreased Gab2-mediated tumor cell migration and invasive potential. Gab2 overexpression resulted in enhanced tumor growth and metastatic potential in vivo. These studies demonstrate a previously undefined role for Gab2 in melanoma tumor progression and metastasis.
Figures




References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. - PubMed
-
- Liu Y, Rohrschneider LR. The gift of Gab. FEBS Lett. 2002;515:1–7. - PubMed
-
- Gu H, Neel BG. The “Gab” in signal transduction. Trends Cell Biol. 2003;13:122–130. - PubMed
-
- Daly RJ, Gu H, Parmar J, Malaney S, Lyons RJ, Kairouz R, Head DR, Henshall SM, Neel BG, Sutherland RL. The docking protein Gab2 is overexpressed and estrogen regulated in human breast cancer. Oncogene. 2002;21:5175–5181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

