Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation
- PMID: 19342382
- PMCID: PMC2708864
- DOI: 10.1074/jbc.M809787200
Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation
Erratum in
-
Correction: Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation.J Biol Chem. 2019 Jun 21;294(25):10018. doi: 10.1074/jbc.AAC119.009552. J Biol Chem. 2019. PMID: 31227623 Free PMC article. No abstract available.
Abstract
Bone tissue arises from mesenchymal cells induced into the osteoblast lineage by essential transcription factors and signaling cascades. MicroRNAs regulate biological processes by binding to mRNA 3'-untranslated region (UTR) sequences to attenuate protein synthesis. Here we performed microRNA profiling and identified miRs that are up-regulated through stages of osteoblast differentiation. Among these are the miR-29, miR-let-7, and miR-26 families that target many collagens and extracellular matrix proteins. We find that miR-29b supports osteoblast differentiation through several mechanisms. miR-29b decreased and anti-miR-29b increased activity of COL1A1, COL5A3, and COL4A2 3'-UTR sequences in reporter assays, as well as endogenous gene expression. These results support a mechanism for regulating collagen protein accumulation during the mineralization stage when miR-29b reaches peak levels. We propose that this mechanism prevents fibrosis and facilitates mineral deposition. Our studies further demonstrate that miR-29b promotes osteogenesis by directly down-regulating known inhibitors of osteoblast differentiation, HDAC4, TGFbeta3, ACVR2A, CTNNBIP1, and DUSP2 proteins through binding to target 3'-UTR sequences in their mRNAs. Thus, miR-29b is a key regulator of development of the osteoblast phenotype by targeting anti-osteogenic factors and modulating bone extracellular matrix proteins.
Figures
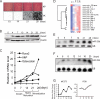
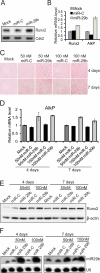


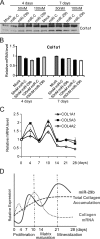
References
-
- Lian J. B., Stein G. S., Javed A., van Wijnen A. J., Stein J. L., Montecino M., Hassan M. Q., Gaur T., Lengner C. J., Young D. W. ( 2006) Rev. Endocr. Metab. Disord. 7, 1– 16 - PubMed
-
- Komori T. ( 2006) J. Cell. Biochem. 99, 1233– 1239 - PubMed
-
- Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J. M., Behringer R. R., de Crombrugghe B. ( 2002) Cell 108, 17– 29 - PubMed
-
- Bodine P. V., Komm B. S. ( 2006) Rev. Endocr. Metab. Disord. 7, 33– 39 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

