The assembled structure of a complete tripartite bacterial multidrug efflux pump
- PMID: 19342493
- PMCID: PMC2678420
- DOI: 10.1073/pnas.0900693106
The assembled structure of a complete tripartite bacterial multidrug efflux pump
Abstract
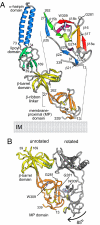
Bacteria like Escherichia coli and Pseudomonas aeruginosa expel drugs via tripartite multidrug efflux pumps spanning both inner and outer membranes and the intervening periplasm. In these pumps a periplasmic adaptor protein connects a substrate-binding inner membrane transporter to an outer membrane-anchored TolC-type exit duct. High-resolution structures of all 3 components are available, but a pump model has been precluded by the incomplete adaptor structure, because of the apparent disorder of its N and C termini. We reveal that the adaptor termini assemble a beta-roll structure forming the final domain adjacent to the inner membrane. The completed structure enabled in vivo cross-linking to map intermolecular contacts between the adaptor AcrA and the transporter AcrB, defining a periplasmic interface between several transporter subdomains and the contiguous beta-roll, beta-barrel, and lipoyl domains of the adaptor. With short and long cross-links expressed as distance restraints, the flexible linear topology of the adaptor allowed a multidomain docking approach to model the transporter-adaptor complex, revealing that the adaptor docks to a transporter region of comparative stability distinct from those key to the proposed rotatory pump mechanism, putative drug-binding pockets, and the binding site of inhibitory DARPins. Finally, we combined this docking with our previous resolution of the AcrA hairpin-TolC interaction to develop a model of the assembled tripartite complex, satisfying all of the experimentally-derived distance constraints. This AcrA(3)-AcrB(3)-TolC(3) model presents a 610,000-Da, 270-A-long efflux pump crossing the entire bacterial cell envelope.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


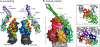

Comment in
-
Trinity revealed: Stoichiometric complex assembly of a bacterial multidrug efflux pump.Proc Natl Acad Sci U S A. 2009 Apr 28;106(17):6893-4. doi: 10.1073/pnas.0902837106. Epub 2009 Apr 22. Proc Natl Acad Sci U S A. 2009. PMID: 19416927 Free PMC article. No abstract available.
References
-
- Koronakis V, Eswaran J, Hughes C. Structure and function of TolC: The bacterial exit duct for proteins and drugs. Annu Rev Biochem. 2004;73:467–489. - PubMed
-
- Eswaran J, Koronakis E, Higgins MK, Hughes C, Koronakis V. Three's company: Component structures bring a closer view of tripartite drug efflux pumps. Curr Opin Struct Biol. 2004;14:741–747. - PubMed
-
- Nikaido H, Zgurskaya HI. AcrAB and related multidrug efflux pumps of Escherichia coli. J Mol Microbiol Biotechnol. 2001;3:215–218. - PubMed
-
- Poole K. Multidrug resistance in Gram-negative bacteria. Curr Opin Microbiol. 2001;4:500–508. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

