Apicomplexan parasites co-opt host calpains to facilitate their escape from infected cells
- PMID: 19342550
- PMCID: PMC3391539
- DOI: 10.1126/science.1171085
Apicomplexan parasites co-opt host calpains to facilitate their escape from infected cells
Abstract
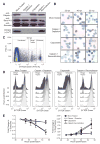
Apicomplexan parasites, including Plasmodium falciparum and Toxoplasma gondii (the causative agents of malaria and toxoplasmosis, respectively), are responsible for considerable morbidity and mortality worldwide. These pathogenic protozoa replicate within an intracellular vacuole inside of infected host cells, from which they must escape to initiate a new lytic cycle. By integrating cell biological, pharmacological, and genetic approaches, we provide evidence that both Plasmodium and Toxoplasma hijack host cell calpain proteases to facilitate parasite egress. Immunodepletion or inhibition of calpain-1 in hypotonically lysed and resealed erythrocytes prevented the escape of P. falciparum parasites, which was restored by adding purified calpain-1. Similarly, efficient egress of T. gondii from mammalian fibroblasts was blocked by either small interfering RNA-mediated suppression or genetic deletion of calpain activity and could be restored by genetic complementation.
Figures


References
-
- Glushakova S, Yin D, Li T, Zimmerberg J. Curr Biol. 2005;15:1645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

