The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells
- PMID: 19342636
- PMCID: PMC2989878
- DOI: 10.4049/jimmunol.0801279
The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells
Abstract
The endogenous purine nucleoside adenosine is an important antiinflammatory mediator that contributes to the control of CD4(+) T cell responses. While adenosine clearly has direct effects on CD4(+) T cells, it remains to be determined whether actions on APC such as dendritic cells (DC) are also important. In this report we characterize DC maturation and function in BMDC stimulated with LPS in the presence or absence of the nonselective adenosine receptor agonist NECA (5'-N-ethylcarboxamidoadenosine). We found that NECA inhibited TNF-alpha and IL-12 in a concentration-dependent manner, whereas IL-10 production was increased. NECA-treated BMDC also expressed reduced levels of MHC class II and CD86 and were less effective at stimulating CD4(+) T cell proliferation and IL-2 production compared with BMDC exposed to vehicle control. Based on real-time RT-PCR, the A(2A) adenosine receptor (A(2A)AR) and A(2B)AR were the predominant adenosine receptors expressed in BMDC. Using adenosine receptor subtype selective antagonists and BMDC derived from A(2A)AR(-/-) and A(2B)AR(-/-)mice, it was shown that NECA modulates TNF-alpha, IL-12, IL-10, and CD86 responses predominantly via A(2B)AR. These data indicate that engagement of A(2B)AR modifies murine BMDC maturation and suggest that adenosine regulates CD4(+) T cell responses by selecting for DC with impaired immunogencity.
Figures
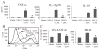


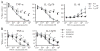



References
-
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. - PubMed
-
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. - PubMed
-
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. - PubMed
-
- Lukashev DE, Smith PT, Caldwell CC, Ohta A, Apasov SG, Sitkovsky MV. Analysis of A2a receptor-deficient mice reveals no significant compensatory increases in the expression of A2b, A1, and A3 adenosine receptors in lymphoid organs. Biochem Pharmacol. 2003;65:2081–2090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI079145/AI/NIAID NIH HHS/United States
- AI070491/AI/NIAID NIH HHS/United States
- P30 DK067629/DK/NIDDK NIH HHS/United States
- U01 AI070491/AI/NIAID NIH HHS/United States
- DK67629/DK/NIDDK NIH HHS/United States
- R01 HL056111/HL/NHLBI NIH HHS/United States
- T32 AI007046/AI/NIAID NIH HHS/United States
- AI069880/AI/NIAID NIH HHS/United States
- P01 HL073361/HL/NHLBI NIH HHS/United States
- U01 AI075526/AI/NIAID NIH HHS/United States
- R01 HL037942/HL/NHLBI NIH HHS/United States
- T35 AI060528/AI/NIAID NIH HHS/United States
- R21 AI069880/AI/NIAID NIH HHS/United States
- DK50980/DK/NIDDK NIH HHS/United States
- AI075526/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

