Ste20-related proline/alanine-rich kinase (SPAK) regulated transcriptionally by hyperosmolarity is involved in intestinal barrier function
- PMID: 19343169
- PMCID: PMC2660421
- DOI: 10.1371/journal.pone.0005049
Ste20-related proline/alanine-rich kinase (SPAK) regulated transcriptionally by hyperosmolarity is involved in intestinal barrier function
Abstract
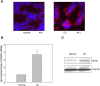
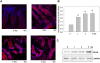
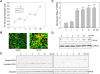
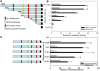
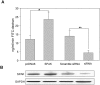
The Ste20-related protein proline/alanine-rich kinase (SPAK) plays important roles in cellular functions such as cell differentiation and regulation of chloride transport, but its roles in pathogenesis of intestinal inflammation remain largely unknown. Here we report significantly increased SPAK expression levels in hyperosmotic environments, such as mucosal biopsy samples from patients with Crohn's disease, as well as colon tissues of C57BL/6 mice and Caco2-BBE cells treated with hyperosmotic medium. NF-kappaB and Sp1-binding sites in the SPAK TATA-less promoter are essential for SPAK mRNA transcription. Hyperosmolarity increases the ability of NF-kappaB and Sp1 to bind to their binding sites. Knock-down of either NF-kappaB or Sp1 by siRNA reduces the hyperosmolarity-induced SPAK expression levels. Furthermore, expression of NF-kappaB, but not Sp1, was upregulated by hyperosmolarity in vivo and in vitro. Nuclear run-on assays showed that hyperosmolarity increases SPAK expression levels at the transcriptional level, without affecting SPAK mRNA stability. Knockdown of SPAK expression by siRNA or overexpression of SPAK in cells and transgenic mice shows that SPAK is involved in intestinal permeability in vitro and in vivo. Together, our data suggest that SPAK, the transcription of which is regulated by hyperosmolarity, plays an important role in epithelial barrier function.
Conflict of interest statement
Figures




Similar articles
-
Nuclear factor-kappaB is a critical mediator of Ste20-like proline-/alanine-rich kinase regulation in intestinal inflammation.Am J Pathol. 2008 Oct;173(4):1013-28. doi: 10.2353/ajpath.2008.080339. Epub 2008 Sep 11. Am J Pathol. 2008. PMID: 18787102 Free PMC article.
-
Characterization of the human intestinal CD98 promoter and its regulation by interferon-gamma.Am J Physiol Gastrointest Liver Physiol. 2007 Feb;292(2):G535-45. doi: 10.1152/ajpgi.00385.2006. Epub 2006 Oct 5. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17023546
-
Overexpression of Ste20-related proline/alanine-rich kinase exacerbates experimental colitis in mice.J Immunol. 2011 Aug 1;187(3):1496-505. doi: 10.4049/jimmunol.1002910. Epub 2011 Jun 24. J Immunol. 2011. PMID: 21705622 Free PMC article.
-
Cloning and characterization of a new intestinal inflammation-associated colonic epithelial Ste20-related protein kinase isoform.Biochim Biophys Acta. 2007 Feb;1769(2):106-16. doi: 10.1016/j.bbaexp.2007.01.003. Epub 2007 Jan 23. Biochim Biophys Acta. 2007. PMID: 17321610 Free PMC article.
-
Ste20-related proline/alanine-rich kinase: a novel regulator of intestinal inflammation.World J Gastroenterol. 2008 Oct 28;14(40):6115-21. doi: 10.3748/wjg.14.6115. World J Gastroenterol. 2008. PMID: 18985800 Free PMC article. Review.
Cited by
-
The choroid plexus links innate immunity to CSF dysregulation in hydrocephalus.Cell. 2023 Feb 16;186(4):764-785.e21. doi: 10.1016/j.cell.2023.01.017. Cell. 2023. PMID: 36803604 Free PMC article.
-
Rho-A prenylation and signaling link epithelial homeostasis to intestinal inflammation.J Clin Invest. 2016 Feb;126(2):611-26. doi: 10.1172/JCI80997. Epub 2016 Jan 11. J Clin Invest. 2016. PMID: 26752649 Free PMC article.
-
SPAK Deficiency Attenuates Chemotherapy-Induced Intestinal Mucositis.Front Oncol. 2021 Nov 23;11:733555. doi: 10.3389/fonc.2021.733555. eCollection 2021. Front Oncol. 2021. PMID: 34888232 Free PMC article.
-
Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport.Physiol Rev. 2012 Oct;92(4):1577-617. doi: 10.1152/physrev.00009.2012. Physiol Rev. 2012. PMID: 23073627 Free PMC article. Review.
-
NF-κB Signaling-Mediated Activation of WNK-SPAK-NKCC1 Cascade in Worsened Stroke Outcomes of Ang II-Hypertensive Mice.Stroke. 2022 May;53(5):1720-1734. doi: 10.1161/STROKEAHA.121.038351. Epub 2022 Mar 11. Stroke. 2022. PMID: 35272484 Free PMC article.
References
-
- Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, et al. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology. 1989;97:927–931. - PubMed
-
- Sandle GI, Higgs N, Crowe P, Marsh MN, Venkatesan S, et al. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97–105. - PubMed
-
- Vernia P, Gnaedinger A, Hauck W, Breuer RI. Organic anions and the diarrhea of inflammatory bowel disease. Dig Dis Sci. 1988;33:1353–1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

