Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state
- PMID: 19343205
- PMCID: PMC2655723
- DOI: 10.1371/journal.ppat.1000360
Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state
Abstract
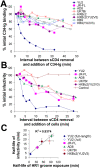
Binding to the CD4 receptor induces conformational changes in the human immunodeficiency virus (HIV-1) gp120 exterior envelope glycoprotein. These changes allow gp120 to bind the coreceptor, either CCR5 or CXCR4, and prime the gp41 transmembrane envelope glycoprotein to mediate virus-cell membrane fusion and virus entry. Soluble forms of CD4 (sCD4) and small-molecule CD4 mimics (here exemplified by JRC-II-191) also induce these conformational changes in the HIV-1 envelope glycoproteins, but typically inhibit HIV-1 entry into CD4-expressing cells. To investigate the mechanism of inhibition, we monitored at high temporal resolution inhibitor-induced changes in the conformation and functional competence of the HIV-1 envelope glycoproteins that immediately follow engagement of the soluble CD4 mimics. Both sCD4 and JRC-II-191 efficiently activated the envelope glycoproteins to mediate infection of cells lacking CD4, in a manner dependent on coreceptor affinity and density. This activated state, however, was transient and was followed by spontaneous and apparently irreversible changes of conformation and by loss of functional competence. The longevity of the activated intermediate depended on temperature and the particular HIV-1 strain, but was indistinguishable for sCD4 and JRC-II-191; by contrast, the activated intermediate induced by cell-surface CD4 was relatively long-lived. The inactivating effects of these activation-based inhibitors predominantly affected cell-free virus, whereas virus that was prebound to the target cell surface was mainly activated, infecting the cells even at high concentrations of the CD4 analogue. These results demonstrate the ability of soluble CD4 mimics to inactivate HIV-1 by prematurely triggering active but transient intermediate states of the envelope glycoproteins. This novel strategy for inhibition may be generally applicable to high-potential-energy viral entry machines that are normally activated by receptor binding.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




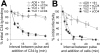

References
-
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. - PubMed
-
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. - PubMed
-
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. - PubMed
-
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

