Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection
- PMID: 19343209
- PMCID: PMC2657210
- DOI: 10.1371/journal.ppat.1000361
Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection
Abstract
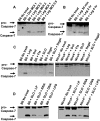
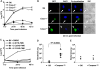
Legionella pneumophila (L. pneumophila), the causative agent of a severe form of pneumonia called Legionnaires' disease, replicates in human monocytes and macrophages. Most inbred mouse strains are restrictive to L. pneumophila infection except for the A/J, Nlrc4(-/-) (Ipaf(-/-)), and caspase-1(-/-) derived macrophages. Particularly, caspase-1 activation is detected during L. pneumophila infection of murine macrophages while absent in human cells. Recent in vitro experiments demonstrate that caspase-7 is cleaved by caspase-1. However, the biological role for caspase-7 activation downstream of caspase-1 is not known. Furthermore, whether this reaction is pertinent to the apoptosis or to the inflammation pathway or whether it mediates a yet unidentified effect is unclear. Using the intracellular pathogen L. pneumophila, we show that, upon infection of murine macrophages, caspase-7 was activated downstream of the Nlrc4 inflammasome and required caspase-1 activation. Such activation of caspase-7 was mediated by flagellin and required a functional Naip5. Remarkably, mice lacking caspase-7 and its macrophages allowed substantial L. pneumophila replication. Permissiveness of caspase-7(-/-) macrophages to the intracellular pathogen was due to defective delivery of the organism to the lysosome and to delayed cell death during early stages of infection. These results reveal a new mechanism for caspase-7 activation downstream of the Nlrc4 inflammasome and present a novel biological role for caspase-7 in host defense against an intracellular bacterium.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


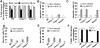
References
-
- Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. - PubMed
-
- Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. - PubMed
-
- Kuribayashi K, Mayes PA, El-Deiry WS. What are caspases 3 and 7 doing upstream of the mitochondria? Cancer Biology & Therapy. 2006;5:763–765. - PubMed
-
- Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. Journal of Biological Chemistry. 1997;272:25719–25723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

